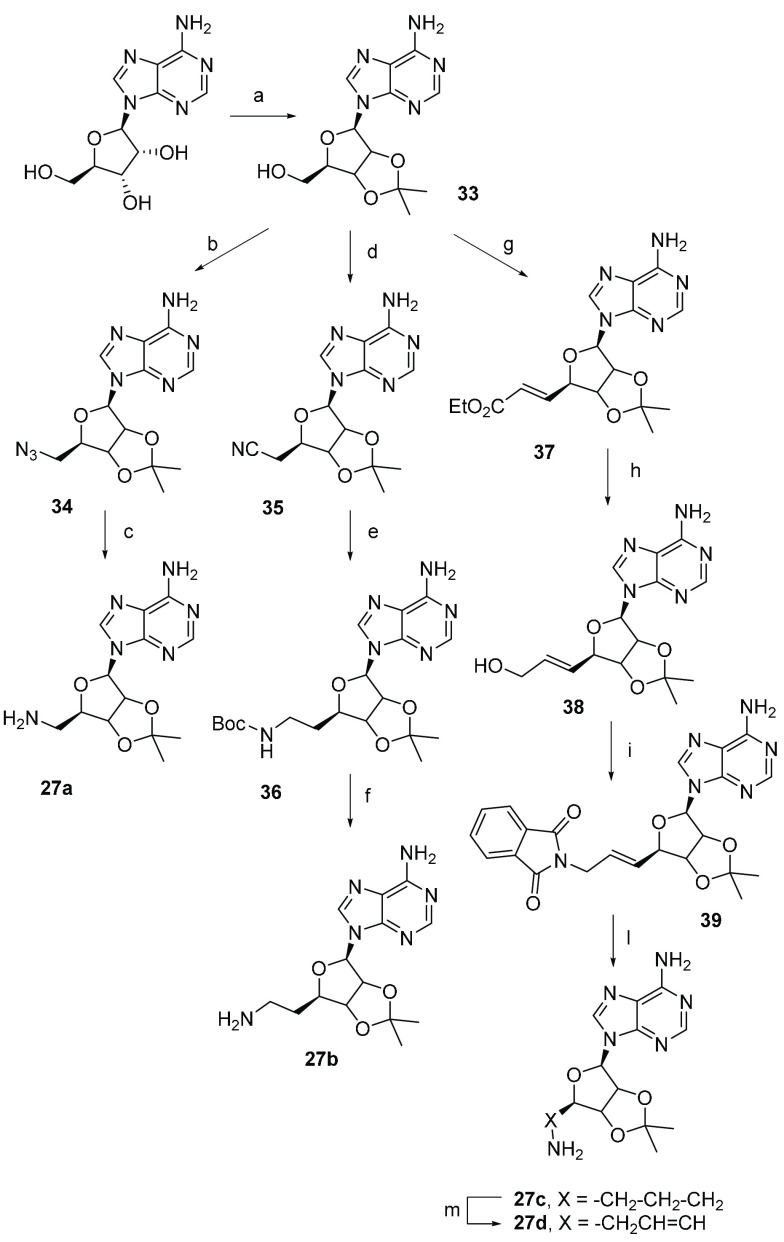
Scheme 5. Synthesis of Derivatives 27a–27d.
Reagents and conditions: (a) acetone, HClO4 70%, r.t., 5 h (81%); (b) NaN3, DPPA, DBU, 15-crown-5, dioxane, 2 h (86%); (c) H2, Pd/C 10%, MeOH, r.t., 5 h (99%); (d) α-hydroxyisobutyronitrile, DEAD, PPh3, dry THF, 0–20 °C, 24 h, (89%); (e) Boc2O, NaBH4, NiCl2·6H2O, dry MeOH, 0 °C, 2 h (80%); (f) DCM/TFA 95:5, t.a, 8 h, (65%); (g) o-iodoxybenzoic acid (IBX), CH3CH2O2CCH=P(C6H5)3, DMSO, 20 °C, 72 h, (70%); (h) DIBAL-H, DCM, −78 °C, 2 h, (98%); (i) phthalimide, DEAD, PPH3, dry THF, 20 °C, 16 h, (70%); (l) NH2NH2·H2O, MeOH, 0–25 °C, 16 h, (90%); (m) H2, Pd/C 10%, AcOEt, 20 °C, 18 h (99%).