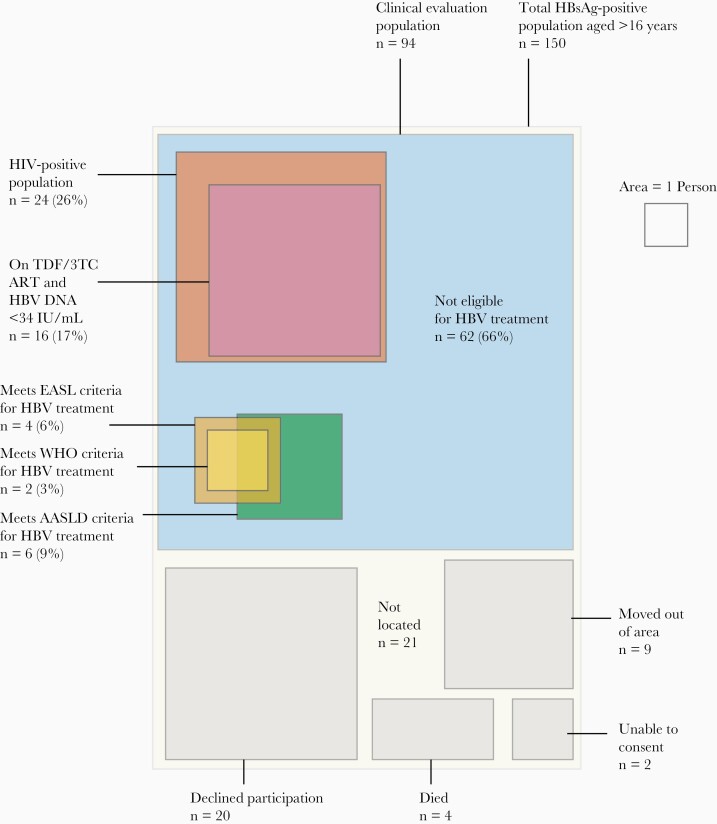
Figure 4.
Outcomes of community clinical evaluation for HBV treatment eligibility. Area is proportional to the number of people in each group. Treatment eligibility criteria are considered only for HIV-negative individuals, as all HIV-positive people should receive ART containing TDF/3TC. Abbreviations: 3TC, lamivudine; AASLD, American Association for the Study of the Liver; ART, antiretroviral therapy; EASL, European Association for Study of the Liver; HBsAg, hepatitis B surface antigen; HBV, hepatitis B virus; HIV, human immunodeficiency virus; TDF, tenofovir disoproxil fumarate; WHO, World Health Organization.