Abstract
In this study, we aimed to assess the quality of life, fatigue, anxiety, and depression after Autologous haematopoietic stem cell transplantation (AHSCT) and to investigate its impact of on separate domains of health status and fatigue in patients with multiple sclerosis (MS). Overall, 18 patients with highly active relapsing MS (mean age 36.3 years, 83.3% female) underwent the AHSCT in Vilnius Multiple Sclerosis center, and we prospectively collected Short Form 36, Health Survey Questionnaire, Fatigue Descriptive Scale, and Hospital Anxiety and Depression Scale beforeand Month3, 12, and 24 after AHSCT. The median score of Expanded Disability Status Scale at Month3 after transplant improved in 14 patients (77.8%). A significant improvement in physical functioning, vitality, and pain was found at Month3 after AHSCT (p < 0.05), which was sustained until Month12 and 24. The improvement in fatigue score was found at Month12 after AHSCT, which was sustained until Month24. Decrease in EDSS score had a positive impact on the better HRQoL outcomes, especially physical and social outcomes. Thus, AHSCT improved quality of life and reduced symptoms of fatigue in patients with highly active relapsing MS. The improvement was determined earlier in the domains of QoL than in the fatigue.
Subject terms: Neurology, Neurological disorders, Multiple sclerosis
Introduction
Multiple sclerosis (MS) is a potentially disabling disease that can cause physical, emotional, and cognitive impairment. Immune ablation followed by autologous hematopoietic stem cell transplantation (AHSCT) has proven to be an effective long term treatment for younger patients with highly active relapsing MS showing clinical and radiological evidence of high disease activity1,2. The results obtained from different studies indicate that up to 86.9% of patients with MS who received AHSCT remained relapse-free, up to 91.3% were EDSS progression-free and 86.3%—MRI event-free3,4.
MS significantly affects patients’ quality of life (QoL), interfering with their ability to work, pursue leisurely activities, and execute daily life tasks5,6. To understand how MS impairs patients’ QoL, the psychological aspects of the disease along with physical aspects and disability should be considered. Several studies have investigated QoL in patients with MS and its’ results vary across regions, cultures and health care systems7,8. Physicians have commonly singled out physical symptoms as the most important negative aspect of this illness, equating health to absence or reduction of disease, and not to complete physical, mental, and social well-being9. However, MS relapses, disability progression and magnetic resonance imaging that reflect the disease activity comprise only part of the impact that MS has on a patient’s daily life10. In recent years, scientists have been recommending the evaluation of health-related quality of life (HRQoL) be included in the definition of No Evidence of Disease Activity11. In line with this, previous studies have shown that patients and physicians disagree on which health domains are most important in MS and its influence on daily life8.
Physical disability only partly impairs QoL in MS. MS-associated fatigue and depression are common and treatable features of the disease, which could also impact on QoL, independent of physical disability12. Fatigue in MS affects up to 90% of patients, and at least one half of them report it as one of their worst symptoms12,13. The pathophysiological causes of the fatigue vary. Mostly, the primary cause of fatigue in MS is the inflammation in the central nervous system12–14, whereas the secondary cause may include fatigue from muscle weakness. Depression, anxiety, and increased attemps in daily activities owing to physical disability also play a role in fatigue15,16. However, fatigue is subjective and varies over the course and severity of the disease, which makes its measurement challenging.
The incidence of psychopathological disorders, especially depression and anxiety, is high in MS, often, higher than in other chronic neurological disorders17,18. Clinical studies suggest that more than half of the patients with MS have symptoms of depression and anxiety, which negatively impacts all HRQoL domains. The depressive symptoms in MS also correlate with fatigue19,20.
Treatment targeting HRQoL and fatique in MS have had mixed levels of success. Some treatments aim to address comorbidities that might contribute to MS such as depression, anxiety, and spasticity or pain treatment through various pharmacologic mechanisms. There is also data showing that disease modification thorough immune modulation or suppression can improve fatigue, reduce anxiety, and improve QoL in patients with MS. During the last 30 years, autologous hematopoetic stem cell transplantation (AHSCT) was proposed as a treatment for severe autoimmune diseases and is a promising alternative treatment for highly active MS21,22.
Since AHSCT is an innovative therapy in the treatment of highly active MS, there is an urgent need for studies which can assess not only the effectiveness and safety of the treatment, but also its impact on the patients' QoL, fatigue, anxiety and depression. Further data is being provided regarding AHSCT in MS treatment; however, despite the wide recognition of the importance of evaluating the emotional aspects, most studies apply the clinical approach.
The study aimed to assess the QoL, fatigue, anxiety and depression after AHSCT in patients with MS and to investigate the impact of AHSCT on the separate domains of health status and fatigue in these patients after AHSCT.
Results
Patients
Eighteen patients treated with AHSCT were included in the study. HRQoL, depression, anxiety and fatigue were assessed in these patients before AHSCT, and thereafter, at Month 3, 12 and 24. Demographic and clinical characteristics of the patients at baseline are provided in Table 1.
Table 1.
Numerical and percentage distribution of patients with MS undergoing AHSCT, according to demographic and clinical characteristics.
| Demographic and clinical variables | N | % |
|---|---|---|
| Female/male | 15/3 | 83.3%/16.7% |
| Mean age and SD at the beginning of the study (years) | 35.2 ± 9.1 | – |
| Mean time of education and SD (years) | 16.3 ± 3.4 | – |
| Disease duration (years) | 8.6 ± 5.5 | |
| Median EDSS score before AHSCT (IQR) | 6.0 (6.0–6.5) | |
| Previous DMT | ||
| Fingolimod | 8 | |
| Natalizumab | 8 | |
| Alemtuzumab | 1 | |
| Interferon-beta | 1 | |
AHSCT Autologous Haematopoietic Stem Cell Transplantation, EDSS Expanded Disability Status Scale, SD standard deviation, IQR interquartile range, DMT disease modifying treatment.
Two patients of 18 (11.1%) had one relapse and one patient of 18 patients (5.6%) had two relapses during the first year after AHSCT. One patient of 18 (5.6%) had one relapse and one patient of 18 (5.6%) had two relapses during the second year after AHSCT. The annualized relapse rate dropped from 2.7 before AHSCT to 0.17 in the first year and to 0.11 in the senond year after AHSCT.
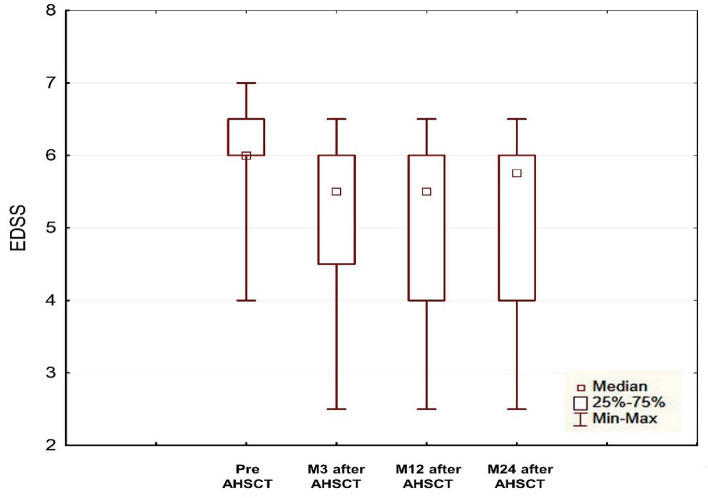
Comparing the mean score on the Expanded Disability Status Scale (EDSS) at the four time points (preAHSCT, M3, M12 and M24), significant improvement was assessed at Month3 after AHSCT (p < 0.05) and EDSS score remained stable at Months 12 and 24 (Fig. 1). The median EDSS score at Month 3 with a minimal change from baseline of 0.5 points improved after transplant in 77.8% of patients; however, in 22.8% of patients EDSS, did not change. Sustained disability improvement was found in 12 (66.7%) of 18 patients at Month 12 and in 11 (61.1%) of 18 patients at Month 24.
Figure 1.
Changes in EDSS score from baseline to Month24 after AHSCT. EDSS Expanded Disability Status Scale, AHSCT autologous hematopoietic stem cell transplantation, preAHSCT before autologous hematopoietic stem cell transplantation, M3 month 3, M12 month 12, M24 month 24.
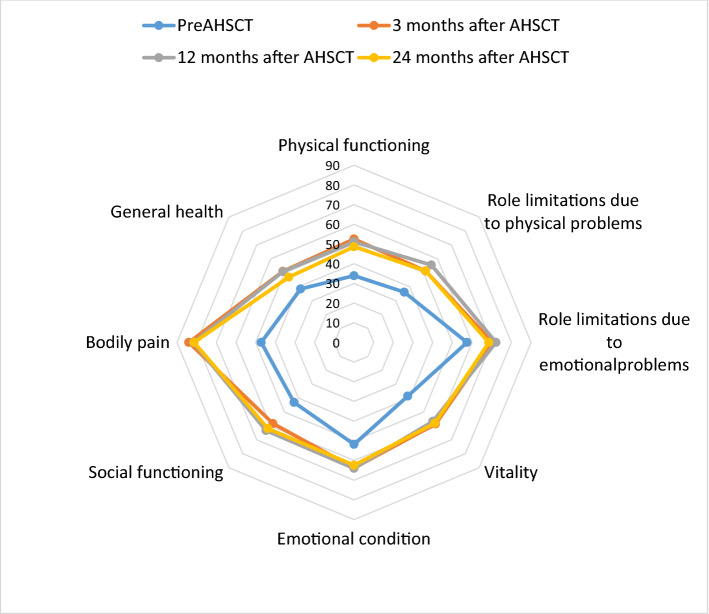
An improvement in all domains of Short Form 36 (SF-36) Health Survey Questionnaire was observed at the first follow-up, that is, Month3 after AHSCT. The improvement was sustained until Month 12 and 24 (Fig. 2).
Figure 2.
HRQoL in patients with MS before and at Month 3, Month 12 and Month 24 after AHSCT. Scored on a 0–100 scale (0 = low HRQoL; 100 = high QoL). AHSCT autologous haematopoietic stem cell transplantation, PreAHSCT before autologous haematopoietic stemm cell transplantion, HRQoL health-related quality of life.
A significant improvement was observed in physical functioning, vitality, and pain at Month 3 after AHSCT (p < 0.05) (Table 2). The median physical functioning score improved by 18.4, vitality by 20 and pain by 36.7 at first follow-up after AHSCT (p < 0.05), which was sustained until the end of the study. In 22.8% of patients, who did not show improvement in EDSS score, the scores of physical functioning and vitality did not change after AHSCT. In this patients, the significant improvement in pain score was observed at Month12 after AHSCT (p < 0.05): the mean score improved by 51.8 at Month 12 compared with the baseline.
Table 2.
The scores of SF-36 domains before AHSCT and 2 years after AHSCT in patients with MS.
| Test | M0 | M3 | M12 | M24 | rmANOVA | Post-hoc |
|---|---|---|---|---|---|---|
| Physical functioning | 34.1 ± 18.5 | 52.5 ± 22.0 | 51.1 ± 23.4 | 48.3 ± 25.9 | F = 6.91; p < 0.05 |
M0 < M3, M12, M24 M3 = M12 = M24 |
| Role limitations due to physical problems | 36.1 ± 37.6 | 51.4 ± 44.1 | 55.6 ± 43.3 | 51.4 ± 46.6 | F = 0.86; p > 0.05 | M0 = M3 = M12 = M24 |
| Role limitations due to emotional problems | 424.1 ± 1558.6 | 70.4 ± 39.4 | 72.2 ± 40.0 | 68.5 ± 38.7 | F = 0.53; p > 0.05 | M0 = M3 = M12 = M24 |
| Vitality | 38.6 ± 12.8 | 58.6 ± 16.1 | 56.7 ± 17.7 | 58.1 ± 19.9 | F = 15.6; p < 0.001 |
M0 < M3, M12, M24 M3 = M12 = M24 |
| Emotional condition | 51.8 ± 6.2 | 63.8 ± 21.0 | 64.0 ± 18.0 | 62.4 ± 18.8 | F = 2.0; p > 0.05 | M0 = M3 = M12 = M24 |
| Social functioning | 43.1 ± 29.1 | 58.3 ± 29.2 | 63.2 ± 27.6 | 61.9 ± 30.1 | F = 2.6; p > 0.05 | M0 = M3 = M12 = M24 |
| Pain | 47.2 ± 8.3 | 83.9 ± 20.1 | 81.7 ± 22.8 | 81.3 ± 19.6 | F = 20.6; p < 0.001 |
M0 < M3, M12, M24 M3 = M12 = M24 |
| General Health | 47.2 ± 19.5 | 51.1 ± 19.6 | 51.5 ± 18.5 | 46.9 ± 20.4 | F = 0.9; p > 0.05 | M0 = M3 = M12 = M24 |
rmANOVA—Repeated measures analysis of variance, AHSCT—autologous hematopoietic stem cell transplantation, M0—before AHSCT, M3—month 3 after AHSCT, M12—month 12 after AHSCT, M24—month 24 after AHSCT.
Significant values are in [bold].
9 patients (50.0%) had moderate and 7 patients (38.9%) had severe depression before AHSCT. 13 patients (72.2%) had moderate anxiety and 1 patient (5.6%) had severe anxiety before AHSCT.
Unlike the scores on the HRQoL questionnaires, where significant improvement was found at Month 3, the improvement in fatigue score was found at Month 12 after AHSCT, and this improvement was sustained until Month 24. The improvement in fatigue score was also observed at Month3; however, it was not significant (p > 0.05). The anxiety and depression scores were stable before AHSCT and at Month 3, 12, and 24 after AHSCT (Table 3). In 22.8% of patients, who did not have improvement in their EDSS score, their score on fatigue and anxiety also did not change after AHSCT. However, there was significant improvement in depression score in these patients at Month 12 after AHSCT (p < 0.05): the mean score decreased by 7.25 at Month 12 compared with the baseline.
Table 3.
The scores of fatigue, anxiety and depression before AHSCT to 2 years after AHSCT in patients with MS.
| Test | M0 | M3 | M12 | M24 | rmANOVA | Post-hoc |
|---|---|---|---|---|---|---|
| Fatigue | 7.9 ± 5.8 | 4.6 ± 4.2 | 2.8 ± 3.8 | 3.0 ± 3.7 | F = 7.12; p < 0.01 |
M0 > M12, M24 M0 = M3, M12 = M24 |
| Anxiety | 11.3 ± 2.1 | 12.2 ± 3.1 | 12.1 ± 2.8 | 11.8 ± 2.2 | F = 0.83; p > 0.05 | M0 = M3 = M12 = M24 |
| Depression | 8.3 ± 2.2 | 7.8 ± 2.0 | 7.9 ± 1.9 | 8.4 ± 2.6 | F = 0.40; p > 0.05 | M0 = M3 = M12 = M24 |
rmANOVA—Repeated measures analysis of variance, AHSCT—autologous hematopoietic stem cell transplantation, M0—before AHSCT, M3—month 3 after AHSCT, M12—month 12 after AHSCT, M24—month 24 after AHSCT.
Significant values are in [bold].
Analysis of variables that explained health status in patients with MS during the last assessment after AHSCT
The General Linear Model was used to assess the impact of demographic and disease characteristics, anxiety, depression, and fatigue during the last visit on the separate domains of health status before and after AHSCT in patients with MS. Dependent variables in the models were the scores of SF-36 domains at Month 24. Independent variables (regressors) in the models were age, gender, disease duration, as well as the EDSS, anxiety, depression, and fatigue scores at Month 24.
Regression model that had explained separate domains of health status after AHSCT:
Physical functioning M24 = 64.1 − 17.9 × EDSSM24 + 4.1 × depression M24; R2 = 0.76; p < 0.05.
Role limitations due to physical problems M24 = 80.9 − 30.2 × EDSSM24; R2 = 0.49; p < 0.05.
Emotion conditional M24 = 28.9 + 5.8 × anxiety M24; R2 = 0.51; p < 0.05.
Social functioning M24 = − 19.4 − 13.1 × EDSSM24 + 5.8 × depression M24 + 7.9 × anxiety M24; R2 = 0.57; p < 0.05.
Only significant independent variables (p < 0.05) were included in the models.
Discussion
The primary aim of the study was to assess the QoL, fatigue, anxiety, and depression in patients with MS after AHSCT. Since 1997, a number of trials on AHSCT in patients with MS and its efficacy and safety have been reported21,22. However, only two trials provide their data according to QoL23,24 and one trial on fatigue25 in patients with MS after AHSCT. In this study, we analysed the HRQoL and fatigue owing to MS by following up with 18 patients with MS on whom AHSCT was performed in one center. Moreover, we emphasized, on the impact of AHSCT on health status and fatigue in patients with MS post transplant.
Evidence from multiple studies indicates that AHSCT is a highly effective treatment alternative for highly active relapsing MS26–28. The current analysis shows that in patients with relapsing MS who do not respond to prior high efficacy DMT adequately, AHSCT provides disability stabilization as well as early disability regression26,29. Our results support previous published data, showing the ability of AHSCT to reduce the disability and to sustain the disability stability for long time26–28.
MS is a chronic and potentially disabling disease that significantly affects patients’ QoL. In MS, QoL is impaired partly owing to physical disability, and partly due to MS-associated fatigue, and depression. Mostly, patients with MS require life-long treatments that can cause major side-effects. Assessing the impact of these interventions on QoL and fatigue is important because it highlights the patient’s perspective on the overall effect of the treatment. Our results show that AHSCT improves HRQoL and fatigue in people with highly active relapsing MS. Absolute scores of all SF-36 domains increased at Month 3 after AHSCT and remained stable at Month 12 and 24, whereas the SF-36 domains physical functioning, vitality and pain were significantly affected by the treatment. Unlike other studies that showed significant improvement in all domains of SF-36 one year after AHSCT6,23,24, we found earlier improvement in HRQoL in patients MS. The lack of significance in other domains, like general health, role limitation because of physical and emotional problems, and social functioning, may be related to a relatively small sample size. In contrast to the scores of HRQoL questionnaires, the improvement in fatigue score was found later, and significant improvement was observed at Month 12 after AHSCT, which sustained until Month 24. However, non-significant decline in anxiety score was observed at Month 3, which indicates increased anxiety in patients in the early phase after transplant. Slight decline in anxiety and later improvement in fatigue score suggest that AHSCT using intermediate intensity lymphoablative conditioning regimen may negatively impact anxiety and fatigue in patients with MS in early phases after the AHSCT procedure. Previously published data have proved the negative impact of AHSCT on cognition in the early phase after AHSCT (month 2 and 3 after AHSCT)29–31; however, other comorbidities like fatigue and anxiety in MS are likely to be affected in the early phase after transplantation.
In almost one quarter of patients who showed no improvement in physical disability, the fatigue and anxiety scores also did not improve after AHSCT either. However, we found significant improvement in depression scores at Month 12 after transplant. Our data support that AHSCT has benefits on the disease stability and even can improve other comorbidities.
We also found an association between lower EDSS and improvement in physical and social functioning as well as role limitations owing to physical problems, during the last follow-up after AHSCT. The current analysis shows that in patients with highly active relapsing MS, disability regression is the main value that has a positive impact on HRQoL outcomes, especially physical and social outcomes. We did not find the value that could have a positive impact on fatigue scores after AHSCT; however, previous studies have demonstrated that better social outcomes are associated with reduction in fatigue score25.
The main limitation of this study was the relatively low sample size. Therefore, the results of this study cannot be generalized; further research is needed to replicate these findings. Furthermore, there was no comparative control group and the assessment was performed in one center. However, we did not identify any controlled study, with the exception of one small randomised controlled trial32 in which a comparative group is used to assess the results of AHSCT.
Conclusions
AHSCT improved quality of life and reduced symptoms of fatigue in patients with highly active relapsing MS. The improvement was determined earlier in the domains of QoL than in the fatigue. A significant improvement in physical functioning, vitality, and pain was found at Month 3 after AHSCT (p < 0.05) in patients with MS, and this improvement was sustained until Month 12 and 24. The improvement in fatigue score was found at Month 12 after AHSCT, which was sustained until Month 24. Primarily, reduction of the EDSS score had a positive impact on the better HRQoL outcomes, especially physical and social outcomes.
Methods
The Lithuanian Bioethics Committee approved the study in 2011 (2011-01-27 No.: L-12-01/2), the permission to continue the study was granted by the Lithuanian Bioethics Committee in 2018 (2018-02-22 No.: 6B-18-41). All methods were carried out in accordance with relevant guidelines and regulations. All participants provided written informed consent. The AHSCT procedure is performed at our hospital as routine clinical practice for highly active relapsing patients with MS who do not respond to the second line MS treatment. Highly active MS patients in the case of AHSCT were defined the patients who experienced at least two relapses and had disability progression of at least 2.0 points according EDSS in the last year.
Participants underwent peripheral blood stem cells mobilization: cyclophosphamide was administered, subcutaneous filgrastim was started on day + 7 and peripheral blood stem cell apheresis procedure were targeted on day + 12 after cyclophosphamide. Collected cells were cryopreserved and stored. The conditioning regimen was intravenous cyclophosphamide and rabbit antithymocyte globulin29.
Patient eligibility
The inclusion criteria were: diagnosis of highly active relapsing MS; aged 18 years and over; performed AHSCT and completed SF-36, Hospital Anxiety and Depression Scale (HADS), and Fatigue Descriptive Scale (FDS) questionnaires before AHSCT and during the period defined by the evaluation, and participants willing to cooperate voluntarily with the research.
Neurologic assessment
Neurologic assessment was scheduled for all patients at baseline, Month 3, 12 and 24 after AHSCT. Neurological disability was assessed with EDSS33. Confirmed significant disability change was defined as disability increase from study baseline, measured by EDSS: increase of ≥ 1.0 points if baseline EDSS was ≤ 5.5 points or an ≥ 0.5-point increase if baseline EDSS was > 5.5 points.
QoL
Eighteen patients in the study completed the SF-36 at baseline, Month 3, 12, and 24 after AHSCT. The SF-36 is a well-known, generic health-related QoL questionnaire with 36 items; it is one of the most used questionnaires assessing subjective psychological well-being in different disorders globally. Psychometric analysis of the translated versions shows that SF-36 is a reliable and valid measure in different languages and for different populations. It measures eight domains of health status: physical functioning, physical role limitations, bodily pain, general health perceptions, energy/vitality, social functioning, emotional role limitations, and mental health34,35. The scores are transformed to range from zero, indicating the worst possible health, to 100, indicating the best possible health.
Anxiety and depression assessment
HADS was used to assess anxiety and depression. It was completed at baseline, Month 3, 12, and 24 after AHSCT. HADS is a brief self-administrated two-dimensional questionnaire, which is one of the most widely used tool to screen anxiety and depression among patients with different disorders36. Previous studies have shown that HADS is an appropriate screening instrument for patients with MS37–39. Of the 14 questions—seven reflect anxiety (Hospital Anxiety and Depression Scale—Anxiety [HADA subscale]) whereas the rest reflect depression (Hospital Anxiety and Depression Scale—Depression (HADD subscale)). A total subscale score of > 8 points out of a possible 21 indicate considerable symptoms of depression or anxiety.
Fatigue assessment
The FDS was used for fatigue assessment in patients with MS at baseline and at Month 3, 12, and 24 after AHSCT. FDS is a valid, quick, and easy to conduct tool to evaluate the severity and quality of fatigue in a group of patients suffering from MS40. Lower scores indicate less fatigue-related disability.
Statistical analysis
Data were analysed using statistical software package SPSS (version 20.0 for Windows). Outcomes are reported based on the last follow-up (Month 24) of each visit. Continuous variables were reported as medians and ranges or means and standard deviations, whereas categorical variables were reported as absolute numbers and percent of total patients. General linear model (GLM) repeated measures were used when assessing the data of different domains of QoL, fatigue, anxiety, and depression at different time points (baseline, Month 3, Month 12 and Month 24). To determine the significance of pairwise comparisons in one-way ANOVA, Bonferroni's post hoc test (for equal variances, Levene's test p > 0.05) was used. Mauchly's test of sphericity was used to test whether or not the assumption of sphericity was met in a repeated measures ANOVA. Linear regression was used to assess the impact of different demographic and clinical factors on the disability regression. A significance level p < 0.05 was accepted.
Author contributions
N.G. contributed to the conception and design of the study, acquisition of the data, analysis and interpretation of the data, and drafting of the manuscript. G.G. contributed to the analysis and interpretation of the data, to the conception and design of the study and revision of the manuscript. G.K. contributed to the conception and design of the study, analysis and interpretation of the data, and drafting of the manuscript. All of the authors discussed the results and contributed to and approved the final manuscript.
Data availability
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bose G, Thebault S, Rush CA, Atkins HL, Freedman MS. Autologous hematopoietic stem cell transplantation for multiple sclerosis: A current perspective. Mult. Scler. 2021;27(2):167–173. doi: 10.1177/1352458520917936. [DOI] [PubMed] [Google Scholar]
- 2.Cencioni MT, Genchi A, Brittain G, et al. Immune reconstitution following autologous hematopoietic stem cell transplantation for multiple sclerosis: A review on behalf of the EBMT autoimmune diseases working party. Front. Immunol. 2022;12:813957. doi: 10.3389/fimmu.2021.813957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nash RA, Hutton GJ, Racke MK, et al. High-dose immunosuppressive therapy and autologous hematopoietic cell transplantation for relapsing-remitting multiple sclerosis (HALT-MS): A 3-year interim report. JAMA Neurol. 2015;72:159–169. doi: 10.1001/jamaneurol.2014.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nash RA, Hutton GJ, Racke MK, et al. High-dose immunosuppressive therapy and autologous HCT for relapsing-remitting MS. Neurology. 2017;88:842–852. doi: 10.1212/WNL.0000000000003660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rezapour A, Almasian Kia A, Goodarzi S, et al. The impact of disease characteristics on multiple sclerosis patients' quality of life. Epidemiol. Health. 2017;39:e2017008. doi: 10.4178/epih.e2017008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shevchenko JL, Kuznetsov AN, Ionova TI, et al. Long-term outcomes of autologous hematopoietic stem cell transplantation with reduced-intensity conditioning in multiple sclerosis: Physician’s and patient’s perspectives. Ann. Hematol. 2015;94(7):1149–1157. doi: 10.1007/s00277-015-2337-8. [DOI] [PubMed] [Google Scholar]
- 7.Rudick RA, Miller DM. Health-related quality of life in multiple sclerosis: Current evidence, measurement and effects of disease severity and treatment. CNS Drugs. 2008;22(10):827–839. doi: 10.2165/00023210-200822100-00004. [DOI] [PubMed] [Google Scholar]
- 8.Kremenchutzky M, Walt L. Perceptions of health status in multiple sclerosis patients and their doctors. Can. J. Neurol. Sci. 2013;40(2):210–218. doi: 10.1017/S0317167100013755. [DOI] [PubMed] [Google Scholar]
- 9.Bassi M, Falautano M, Cilia S, et al. The coexistence of well- and ill-being in persons with multiple sclerosis, their caregivers and health professionals. J. Neurol. Sci. 2014;337(1–2):67–73. doi: 10.1016/j.jns.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 10.Lysandropoulos AP, Havrdova E, Paradig MSG. “Hidden’ factors influencing quality of life in patients with multiple sclerosis. Eur. J. Neurol. 2015;22(Suppl 2):28–33. doi: 10.1111/ene.12801. [DOI] [PubMed] [Google Scholar]
- 11.Stangel M, Penner IK, Kallmann BA, Lukas C, Kieseier BC. Towards the implementation of ‘no evidence of disease activity’ in multiple sclerosis treatment: The multiple sclerosis decision model. Ther. Adv. Neurol. Disord. 2015;8(1):3–13. doi: 10.1177/1756285614560733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rottoli M, La Gioia S, Frigeni B, Barcella V. Pathophysiology, assessment and management of multiple sclerosis fatigue: An update. Expert Rev. Neurother. 2017;17(4):373–379. doi: 10.1080/14737175.2017.1247695. [DOI] [PubMed] [Google Scholar]
- 13.Alvarenga-Filho H, Papais-Alvarenga RM, Carvalho SR, et al. Does fatigue occur in MS patients without disability? Int. J. Neurosci. 2015;125(2):107–115. doi: 10.3109/00207454.2014.909415. [DOI] [PubMed] [Google Scholar]
- 14.Kantorová E, Poláček H, Bittšanský M, et al. Hypothalamic damage in multiple sclerosis correlates with disease activity, disability, depression, and fatigue. Neuro.l Res. 2017;39(4):323–330. doi: 10.1080/01616412.2016.1275460. [DOI] [PubMed] [Google Scholar]
- 15.Šabanagić-Hajrić S, Suljić E, Kučukalić A. Fatigue during multiple sclerosis relapse and its relationship to depression and neurological disability. Psychiatr. Danub. 2015;27(4):406–412. [PubMed] [Google Scholar]
- 16.Marchesi O, Vizzino C, Meani A, et al. Fatigue in multiple sclerosis patients with different clinical phenotypes: A clinical and magnetic resonance imaging study. Eur. J. Neurol. 2020;27(12):2549–2560. doi: 10.1111/ene.14471. [DOI] [PubMed] [Google Scholar]
- 17.Haussleiter IS, Brüne M, Juckel G. Psychopathology in multiple sclerosis. Ther. Adv. Neurol. Disord. 2009;2(1):13–29. doi: 10.1177/1756285608100325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koohi F, Nedjat S, Yaseri M, Cheraghi Z. Quality of life among general populations of different countries in the past 10 years, with a focus on human development index: A systematic review and meta-analysis. Iran. J. Public Health. 2017;46(1):12–22. [PMC free article] [PubMed] [Google Scholar]
- 19.Hanna M, Strober LB. Anxiety and depression in Multiple Sclerosis (MS): Antecedents, consequences, and differential impact on well-being and quality of life. Mult. Scler. Relat. Disord. 2020;44:102261. doi: 10.1016/j.msard.2020.102261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alsaadi T, Hammasi KE, Shahrour TM, et al. Depression and anxiety as determinants of health-related quality of life in patients with multiple sclerosis—United Arab Emirates. Neurol. Int. 2017;9(4):7343. doi: 10.4081/ni.2017.7343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Atkins HL, Bowman M, Allan D, et al. Immunoablation and autologous haemopoietic stem-cell transplantation for aggressive multiple sclerosis: a multicentre single-group phase 2 trial. Lancet. 2016;388(10044):576–585. doi: 10.1016/S0140-6736(16)30169-6. [DOI] [PubMed] [Google Scholar]
- 22.Gavriilaki M, Sakellari I, Gavriilaki E, et al. Autologous hematopoietic cell transplantation in multiple sclerosis: Changing paradigms in the era of novel agents. Stem Cells Int. 2019;2019:5840286. doi: 10.1155/2019/5840286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Guimarães FA, Oliveira-Cardoso EA, Mastropietro AP, Voltarelli JC, Santos MA. Impact of autologous hematopoetic stem cell transplantation on the quality of life of patients with multiple sclerosis. Arq. Neuropsiquiatr. 2010;68(4):522–527. doi: 10.1590/S0004-282X2010000400009. [DOI] [PubMed] [Google Scholar]
- 24.Saccardi R, Mancardi GL, Solari A, et al. Autologous HSCT for severe progressive multiple sclerosis in a multicenter trial: Impact on disease activity and quality of life. Blood. 2005;105(6):2601–2607. doi: 10.1182/blood-2004-08-3205. [DOI] [PubMed] [Google Scholar]
- 25.Bose G, Atkins HL, Bowman M, Freedman MS. Autologous hematopoietic stem cell transplantation improves fatigue in multiple sclerosis. Mult. Scler. 2019;25(13):1764–1772. doi: 10.1177/1352458518802544. [DOI] [PubMed] [Google Scholar]
- 26.Burt RK, Han X, Quigley K, et al. Real-world application of autologous hematopoietic stem cell transplantation in 507 patients with multiple sclerosis. J. Neurol. 2022;269(5):2513–2526. doi: 10.1007/s00415-021-10820-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Muraro PA, Martin R, Mancardi GL, et al. Autologous haematopoietic stem cell transplantation for treatment of multiple sclerosis. Nat. Rev. Neurol. 2017;13(7):391–405. doi: 10.1038/nrneurol.2017.81. [DOI] [PubMed] [Google Scholar]
- 28.Currò D, Mancardi G. Autologous hematopoietic stem cell transplantation in multiple sclerosis: 20 years of experience. Neurol. Sci. 2016;37(6):857–865. doi: 10.1007/s10072-016-2564-3. [DOI] [PubMed] [Google Scholar]
- 29.Giedraitiene N, Kizlaitiene R, Peceliunas V, et al. Selective cognitive dysfunction and physical disability improvement after autologous hematopoietic stem cell transplantation in highly active multiple sclerosis. Sci. Rep. 2020;10(1):21286. doi: 10.1038/s41598-020-78160-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Walker LAS, Berard JA, Atkins HL, et al. Cognitive change and neuroimaging following immunoablative therapy and hematopoietic stem cell transplantation in multiple sclerosis: A pilot study. Mult. Scler. Relat. Disord. 2014;3(1):129–135. doi: 10.1016/j.msard.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 31.Berard JA, Bowman M, Atkins HL, Freedman MS, Walker LAS. Cognitive fatigue in individuals with multiple sclerosis undergoing immunoablative therapy and hematopoietic stem cell transplantation. J. Neurol. Sci. 2014;336(1–2):132–137. doi: 10.1016/j.jns.2013.10.023. [DOI] [PubMed] [Google Scholar]
- 32.Burt RK, Balabanov R, Burman J, et al. Effect of nonmyeloablative hematopoietic stem cell transplantation vs continued disease-modifying therapy on disease progression in patients with relapsing-remitting multiple sclerosis: A randomized clinical trial. JAMA. 2019;321(2):165–174. doi: 10.1001/jama.2018.18743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS) Neurology. 1983;33:1444–1452. doi: 10.1212/WNL.33.11.1444. [DOI] [PubMed] [Google Scholar]
- 34.Ware JE, Brook RH, Williams KN, et al. Conceptualisation and Measurement of Health for Adults in the Health Insurance Study, Vol. 1. Model of Health and Methodology (Santa Monica, CAR and Corp, 1980).
- 35.Ware JE, Jr, Sherbourne CD. The MOS 36-Item Short-Form Health Survey (SF-36): Conceptual framework and item selection. Med. Care. 1992;30:473–481. doi: 10.1097/00005650-199206000-00002. [DOI] [PubMed] [Google Scholar]
- 36.Pais-Ribeiro JL, Martins da Silva A, Vilhena E, et al. The hospital anxiety and depression scale, in patients with multiple sclerosis. Neuropsychiatr. Dis. Treat. 2018;14:3193–3197. doi: 10.2147/NDT.S184260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson TM, Ford E, Worthington E, Lincoln NB. Validation of mood measures for people with multiple sclerosis. Int. J. MS Care. 2014;16(2):105–109. doi: 10.7224/1537-2073.2013-013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Litster B, Fiest KM, Patten SB, et al. Screening tools for anxiety in people with multiple sclerosis. Int. J. MS Care. 2016;18(6):273–281. doi: 10.7224/1537-2073.2016-004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hind D, Kaklamanou D, Beever D, et al. The assessment of depression in people with multiple sclerosis: A systematic review of psychometric validation studies. BMC Psychiatry. 2016;16:278. doi: 10.1186/s12888-016-0931-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iriarte J, Katsamakis G, Castro P. The Fatigue Descriptive Scale (FDS): A useful tool to evaluate fatigue in multiple sclerosis. Mult. Scler. 1999;5(1):10–16. doi: 10.1177/135245859900500103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analysed during the current study available from the corresponding author on reasonable request.