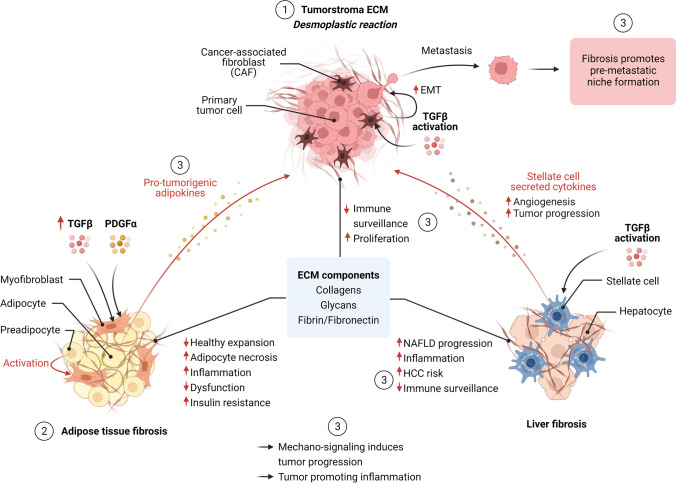
Fig. 1.
Overview of fibrosis in cancer and obesity. (1) ECM remodeling and fibrosis in cancer: The development of a fibrotic tumor stroma (desmoplastic reaction) is a multistep process in part driven by activation of cancer-associated fibroblasts (CAFs) and TGFbeta signaling. CAFs and ECM components exert different pro-tumorigenic processes including epithelial-mesenchymal transition (EMT) in tumor cells, thereby inducing tumor progression (metastasis formation). A fibrotic tumor stroma prevents immune cell infiltration, thereby reducing immune surveillance and responsiveness to cancer immunotherapy. (2) Adipose tissue fibrosis in obesity: Obesity-driven pathologic expansion of adipose tissue is characterized by adipocyte hypertrophy and cell death, chronic inflammation (including macrophage infiltration), and enhanced fibrosis. Fibrosis limits the expansion of adipose and contributes to metabolic dysfunction, including systemic inflammation and insulin resistance. Elevated TGFβ levels under obesity conditions contribute to adipose tissue fibrosis and inflammation. PDGF signaling induces a myofibroblast-like phenotype in a subset of adipocyte progenitors contributing to ECM deposition. (3) ECM remodeling and fibrosis linking metabolism and cancer: Mammary adipose tissue fibrosis contributes to malignant transformation and breast cancer progression through various mechanisms including collagen-derived endotrophin, increased tissue stiffness activating mechano-sensitive pathways, and induced inflammation (e.g., inducing a tumor-promoting macrophage phenotype). The degree of liver fibrosis is a strong predictor of NAFLD progression towards HCC. Activation of stellate cells (myofibroblast phenotype) by TGFβ and other cytokines mainly contributes to hepatic fibrosis. Stellate cell-secreted cytokines (including TGFbeta, PDGF, and VEGF) induce angiogenesis, reduced immune surveillance, and HCC progression. Obesity-induced pancreatic inflammation and desmoplasis contribute to PDAC progression and chemotherapy resistance