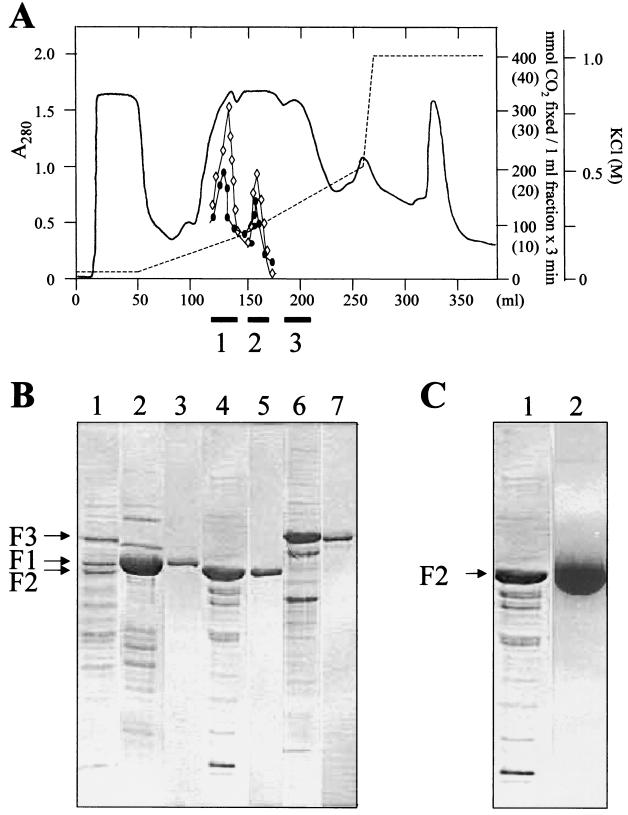
FIG. 3.
Partial purification of phenol-induced proteins. (A) DEAE-Sepharose fast-flow chromatography of 15 ml of the soluble fraction (supernatant obtained after centrifugation at 105 × g) of T. aromatica grown on phenol and nitrate. Fractions containing the F1, F2, and F3 proteins (identified by SDS-PAGE) that were pooled are indicated as black bars (1, 2, and 3, respectively) below the x axis. Only 10 to 20% of both the phenylphosphate carboxylating activity and the isotope exchange were detectable in the F1 and F2 pools. In the F3 pool, none of the activities were measurable. Circle, phenylphosphate carboxylase activity (values in parentheses). Rhombus, isotope-exchange activity. (B) SDS–10% PAGE of the fractions obtained by chromatography of the soluble protein fraction on a DEAE-Sepharose fast-flow column. Lane 1, extract of cells grown with phenol and nitrate; lane 2, pooled fractions containing F1; lane 4, pooled fractions containing F2; lane 6, pooled fractions containing F3; lanes 3, 5, and 7, F1 to F3 were further purified by chromatography on MonoQ. The three proteins eluted with 60, 200, and 330 mM KCl, respectively. (C) SDS–10% PAGE of F2 (fractionated by DEAE-Sepharose fast flow) after chromatography on blue Sepharose. Lane 1, DEAE fraction of F2; lane 2, main fraction of F2 eluting with 10 mM phenylphosphate from blue Sepharose.