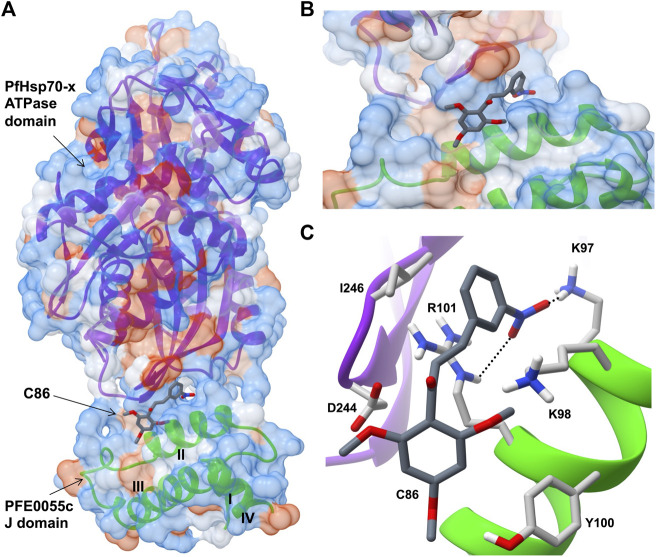
FIGURE 3.
Predicted model of a complex of the small molecule inhibitor C86, PFE0055c J domain and PfHsp70-x ATPase domain. (A) Overall binding pose of C86 (middle) to the complex formed between the PfHsp70-x ATPase domain (top) and the PFE0055c J domain (bottom). (B) Focused view of c86 binding to the complex. (C) Detailed view of residues interacting with C86. The surface (set at 60% transparency) is colored according to amino acid hydrophobicity using the Kyte-Doolittle scale (with dodger blue representing more hydrophilic, to white, to orange red representing more hydrophobic residues). The cartoon representation underneath the surface depicts the secondary structure elements in the PfHsp70-x ATPase domain (purple) and PFE0055c J domain (green). The roman numerals (I to IV) mark the four helices of the PFE0055c J domain. C86 as well the interacting residues of the complex have been shown using the element color scheme (using red, blue and grey for oxygen, nitrogen and carbon, respectively). The carbons in C86 are depicted using dark grey while its interacting residues are colored in light grey. The residues are shown as sticks, and the numbering refers to their position in the full length protein. The predicted hydrogen bonds were identified using LigPlot + version 2.2.5 (https://www.ebi.ac.uk/thornton-srv/software/LigPlus/; Laskowski and Swindells, 2011), and have been rendered using FindHBond option in Chimera in relaxed mode and depicted using dotted lines (black). The lengths of the larger and the smaller hydrogen bonds are 2.74 Å and 2.32 Å, respectively. The PfHsp70-x ATPase domain-PFE0055c J domain complex was obtained using HADDOCK2.2 (Dutta et al., 2020a) while AutoDock Vina (https://vina.scripps.edu/; Trott and Olson, 2010) was used to dock C86 into this complex. Images for the 3D structures were rendered using UCSF Chimera 1.10.1 (https://www.cgl.ucsf.edu/chimera/), using one of the nine best binding conformations of C86.