FIGURE 1.
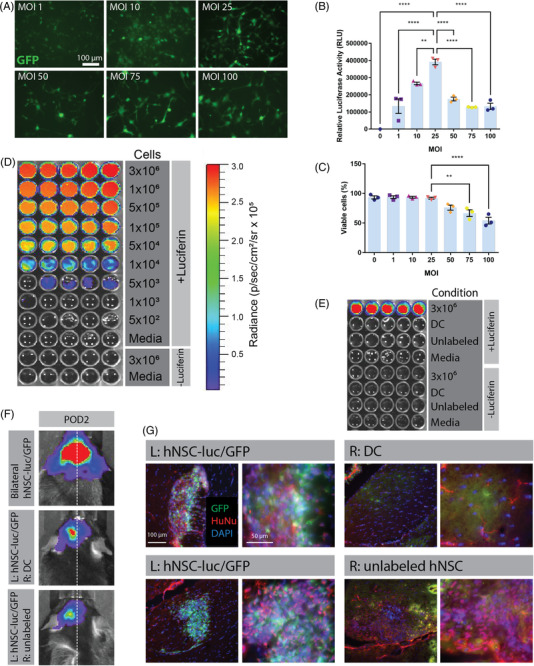
Development and validation of bioluminescent imaging (BLI) to assess transplanted human neural stem cell (hNSC) graft viability in vivo. (A) Fluorescence microscopy of green fluorescent protein (GFP) expression in hNSCs modified to express a dual reporter luc+/GFP+ vector at increasing multiplicity of infection (MOI) 48 h post‐transduction. Luciferase assay (B) and trypan blue exclusion viability assay (C) of hNSC‐luc+/GFP+ performed at 72 h post‐transduction. (D) In vitro BLI of hNSC‐luc+/GFP+ cells at concentrations ranging from 3 × 106 to 5 × 102 per well, with no luciferin and media only controls. (E) In vitro BLI of unlabelled hNSC and dead hNSC‐luc+/GFP+ (DC), with hNSC‐luc+/GFP+ cells as a positive control. (F) In vivo BLI detection of transplanted hNSC‐luc+/GFP+ in 8‐week‐old C57BL/6J mice on post‐operative day (POD) 2 after bilateral injection of 3.6 × 105 hNSC‐luc+/GFP+, or unilateral injection of 1.8 × 105 hNSC‐luc+/GFP+ (L: left side) with contralateral injection of 1.8 × 105 DC or unlabelled hNSC transplants (n = 2 per group). (G) Representative POD 2 immunohistochemical (IHC) images showing the fimbria fornix target area in C57BL/6J mice, with hNSC‐luc+/GFP+ grafts expressing GFP (green) and human‐specific nuclear antibody HuNu (red), with contralateral staining of DC or unlabelled hNSC. Data presented as mean ± standard error of the mean (S.E.M.) for luciferase activity and cell viability analysed by ANOVA with Tukey's post‐test for comparisons of multiple groups. **p < .01; ****p < .0001. DC, dead cells; POD, post‐operative day
