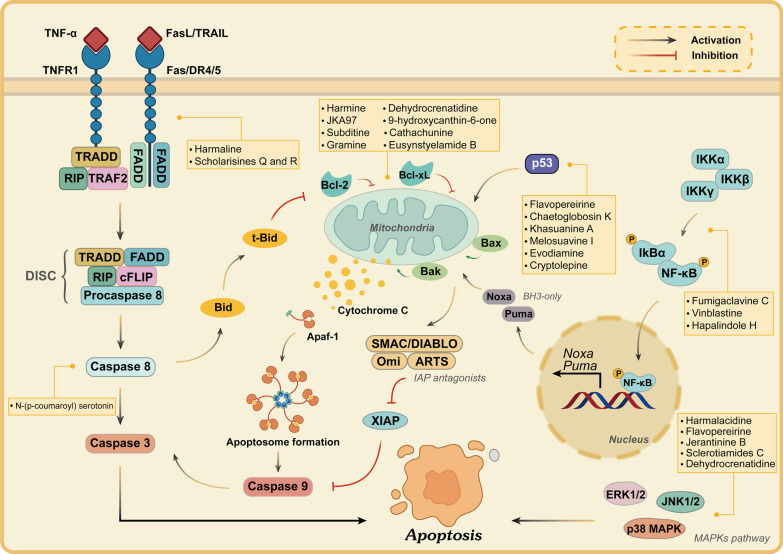
Fig. 4.
Core apoptosis signaling pathway in cancer. Apoptosis is activated by two pathways, the extrinsic and intrinsic pathways. The extrinsic pathway is triggered by death receptors such as TNFR1, Fas, and death receptor (DR) 4/5 by their related ligands TNF-α, Fas ligand (FasL), and TNF-related apoptosis-inducing ligand (TRAIL). Ligand binding to these receptors leads to the recruitment of FADD and TRADD, and then forms a complex called death-inducing signaling complex (DISC), which in turn results in the cleavage of procaspase-8 and sends a signal to activate caspase-8, thus activating caspase-3, and ultimately leads to apoptosis. The intrinsic pathway is stimulated by cellular stress, leading to p53 and BH3 only proteins activation, which in turn induces Bak/Bax oligomerization and permeabilization of the mitochondria, and ultimately promotes the release of cytochrome C (Cyt-C). Cyt-C forms a complex with apoptotic protease activating factor 1 (Apaf-1) and procaspase-9 and then activates caspase-9. Caspase-9 triggers the caspase-3 activation and induces apoptotic cell death. Besides, the inhibitor of apoptosis protein (IAP) family negatively regulates caspase activation and can be inhibited by second mitochondria-derived activator of caspase (SMAC)/direct IAP-binding protein with low pI (DIABLO), Omi, and apoptosis-related protein in the TGF-β signaling pathway (ARTS). Under certain conditions, cross talk from the extrinsic pathway via caspase-8-mediated truncation of Bid to t-Bid can also cause mitochondrial permeabilization. Additionally, the mitogen-activated protein kinase (MAPK) and nuclear factor kappa-B (NF-κB) pathways also play essential roles in regulating apoptotic cell death