Abstract
Microglia are the resident immune cells in the brain. It is well known that brain injury can activate the microglia and induce its directional migration towards the injury sites for exerting immune functions. While extracellular ATP released from the injury site mediates the directionality of activated microglia's migration, what endows activated microglia with migration capability remains largely unexplored. In the present study, we used the larval zebrafish as an in vivo model to visualize the dynamics of both morphology and Ca2+ activity of microglia during its migration evoked by local brain injury. We found that, in response to local injury, activated microglia exhibited an immediate Ca2+ transient and later sustained Ca2+ bursts during its migration towards the local injury site. Furthermore, suppression of Ca2+ activities significantly retarded microglial cell migration. Thus, our study suggests that intracellular Ca2+ activity is required for activated microglia's migration.
Keywords: Microglia, Migration, Ca2+ activity, IP3 receptor, Two-photon imaging, Zebrafish
Highlights
-
•
Larval zebrafish was used to in vivo visualize injury-induced microglia migration.
-
•
An elevated Ca2+ activity is correlated with microglia migration.
-
•
The Ca2+ activity depends on IP3Rs and is required for microglia migration.
1. Introduction
Microglia are primary immune cells in the brain and derived from a myeloid lineage in the mesoderm [[1], [2], [3]]. Its high sensitivity to ATP and other injury-relevant molecules enables it to rapidly detect and be activated by brain injury and inflammation [[4], [5], [6]]. Besides its activated state for immune functions, intensive studies illustrate microglia at the resting state as a multifunction player for regulating diverse neural processes, including synapse pruning [7], myelin formation [8], neuronal activity homeostasis [9], learning [10], forgetting [11], and neuronal death [12,13].
Brain injury can alter microglia status from a physiological resting state to an activated state followed by directional migration towards the injury site [2]. It has been discovered that this process is dependent on injury site-released ATP which is sensed by microglia through P2 receptors and that the concentration gradient of ATP can position the injury site to direct microglia migration [[4], [5], [6]]. However, it is still unexplored that what enables activated microglia to migrate. Considering that Ca2+ activity is required for the migration of many types of cells [[14], [15], [16], [17], [18]] and Ca2+ signaling is important for activating microglia [19,20], we speculate that Ca2+ activity plays a role in the migration of activated microglia.
Taking advantage of the optical transparency and small size of the brain in larval zebrafish [21], we performed in vivo time-lapse two-photon imaging and visualized the dynamics of both the morphology and Ca2+ activity of microglia in response to local brain injury mimicked by two-photon laser-based neuron ablation and micropipette stabbing in zebrafish larvae at 5–8 days post-fertilization (dpf). Both types of local brain injury could effectively induce the directional migration of microglia. Interestingly, we found that, during its migration towards the injury site, microglia displayed robust sustained Ca2+ burst activities. We then found that the Ca2+ burst activities of activated microglia could be suppressed by locally applying 2-Aminoethoxydiphenyl Borate (2-APB), a general antagonist of the inositol 1,4,5-trisphosphate receptors (IP3Rs) [22]. Importantly, we found that the speed of microglial cell migration was reduced simultaneously with the suppression of Ca2+ activity, which suggests that the intracellular Ca2+ activity is important for microglia migration induced by brain injury.
2. Methods
2.1. Zebrafish husbandry and transgenic lines
Adult zebrafish were maintained in an automatic housing system (ESEN, Beijing, China) at 28 °C under a 14-hr light:10-hr dark cycle [9]. Embryos were raised up in an incubator. For two-photon imaging, the 0.003% N-Phenylthiourea was used to inhibit pigment formation. The Tg(coro1a:GCaMP5)ion15d line was generated by using the same promoter as the Tg(coro1a:eGFP) line which was previously described [23]. The Tg(HuC:NES-jRGECO1a)ion72d line was previously described [24].
2.2. In vivo time-lapse two-photon imaging
In vivo time-lapse two-photon imaging [25] with a 4–10 s interval was performed on the double transgenic larvae Tg(coro1a:GCaMP5);Tg(HuC:NES-jRGECO1a) at 5–8 dpf at room temperature (20–22 °C). Zebrafish larvae were first paralyzed by α-bungarotoxin and then immobilized in 1.5% low-melting agarose. Imaging was performed under a 25 × water-immersion objective (N.A., 0.8) on an Olympus FVMPE-RS-TWIN microscope equipped with a two-photon laser (Newport/Spectra-Physics, USA) tuned to 1000 nm for excitation of GCaMP5. The GCaMP5 signal was used to characterize the dynamics of both the morphology and Ca2+activity of microglia.
2.3. Two-photon ablation-based local brain injury
The two-photon ablation-based local brain injury was achieved under the Olympus FVMPE-RS-TWIN with an angular tornado-scanning mode. Two-photon laser (800 nm) was targeted on jRGECO1a-positive cells with a circular region of interest (ROI, 5 μm in diameter) in Tg(coro1a:GCaMP5);Tg(HuC:NES-jRGECO1a) larvae. Successful ablation was accepted when the targeted area exhibited a small autofluorescence sphere (5–10 μm in diameter) as described in previous studies [4,26].
2.4. Micropipette-induced local brain injury
Before the injury, the skin on the surface of the hindbrain of zebrafish larvae was dissected. Under FVMPE-RS-TWIN, a glass micropipette (1–2 μm in diameter of the tip open) made from borosilicate glass capillaries (BF100-58-10, Sutter Instrument) was inserted from the surgery site and the tip was placed in the optic tectum cell body layer. After insertion, the micropipette was moved back and forth for around 5–10 μm to induce tissue damage as previously described [4]. The success of injury induction was confirmed by significant microglial cell migration to the injury site.
2.5. Local puff of 2-APB
A stock solution containing 2-APB at 50 mM was first made with DMSO. It was then diluted to 7.5 mM with an extracellular solution (ES) consisted of (in mM): 134 NaCl, 2.9 KCl, 2.1 CaCl2, 1.2 MgCl2, 10 HEPES, and 10 glucose (pH = 7.8), and then loaded into a glass micropipette (1–2 μm in diameter of the tip open). Ten minutes after micropipette-induced injury, 2-APB solution contained in the micropipette was ejected out by pulses of gas pressure (4 psi, 10 ms in duration, 1 min in interval) until the end of imaging. In the control group, exact the same amount of DMSO was diluted in ES and puffed out with the same pulse pattern.
2.6. Data analysis
Analysis of both the Ca2+ activity and morphology of microglia was performed with ImageJ (NIH). The image sequences were projected using Z-projection via stacking all slices to the same X–Y plane by maximum gray values. An ROI was drawn to include all the processes and cell body of each microglial cell. In terms of Ca2+ activity, the mean gray-scale value was represented by F; the averaged gray-scale value of the first 100 values from ascendingly sorted F was calculated as F0. The level of Ca2+ activity was quantified by (F–F0)/F0 and an increase of >3 SD was counted as a Ca2+ transient activity. The distance to the injury site was measured by calculating the distance between the far side of the microglial cell body and the micropipette tip.
2.7. Statistics
All significance calculation was carried out by Student's t-test. Unpaired and paired Student's t-test was performed for calculating the significance between the data obtained from two groups (migrating microglia versus non-migrating microglia and ES versus 2-APB) and from the same group before and after manipulations (before injury versus after injury, before local puff versus after local puff), respectively. The p < 0.05 was considered as statistically significant. Data were represented as mean ± SEM.
3. Results
3.1. Microglia simultaneously displayed directional migration and Ca2+ activities in response to local brain injury in zebrafish in vivo
Firstly, we confirmed the effectiveness of the previously described laser-induced and micropipette-induced brain injury for activating microglia. To visualize the dynamics of microglial morphology, we performed in vivo time-lapse two-photon imaging of the double transgenic larvae Tg(coro1a:GCaMP5);Tg(HuC:NES-jRGECO1a) at 5–8 dpf at 0.1–0.2 Hz (Fig. 1A), in which GCaMP5 (green) and jRGECO1a (red) are expressed specifically in the microglia and neurons of the brain, respectively. Here, GCaMP5 was used to monitor both the morphology and Ca2+ activity of microglia, and jRGECO1a was used for absorbing two-photon laser-associated heat to induce local brain injury in the midbrain optic tectum where microglia enrich. In response to the local injury, microglia nearby in the same hemisphere became activated as indicated by an amoeboid-like shape and migrated towards the injury site (Fig. 1B). With time, more and more microglia were recruited to surround the injury site (right, Fig. 1B). Furthermore, we used a micropipette to induce local brain injury and observed a similar pattern of activated microglia's migration but within a smaller spatial range due to the more restricted injury induced by micropipette than by two-photon laser (Fig. 1C). This phenomenon was observed in most of the cases examined (20 out of 20 cases for two-photon laser and 13 out of 15 cases for micropipette).
Fig. 1.
Local brain injury-induced migration of microglia in larval zebrafish in vivo.
(A) Whole-brain projection images showing the distribution of microglia (GCaMP5-positive, green) and neurons (jRGECO1a-positive, red) in a double transgenic larva Tg(coro1a:GCaMP5);Tg(HuC:NES-jRGECO1a) at 5 dpf. The green signals on the eyeballs were auto-fluorescence. C, caudal; L, lateral. Scale bar, 50 μm
(B) In vivo time-lapse (with a 5-s interval) two-photon images showing microglia's directional migration evoked by two-photon laser-induced local brain injury (red dot). Only time series at a 15-min interval were shown. The time point of 0 min indicates the onset of injury induction. The numbers and arrowheads indicate the microglia traced across the whole imaging. The image field is outlined in (A), and the images were obtained from a 7-dpf larva. Scale bar, 20 μm
(C) In vivo time-lapse (with a 4-s interval) two-photon images showing microglia's directional migration evoked by micropipette (dashed orange lines)-induced local brain injury. Only time series at a 5-min interval were shown. The time point of 0 min indicates the onset of micropipette insertion. The numbers and arrowheads indicate the microglia traced across the whole imaging. The image field is outlined in (A), and the images were obtained from a 5-dpf larva. Scale bar, 10 μm. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
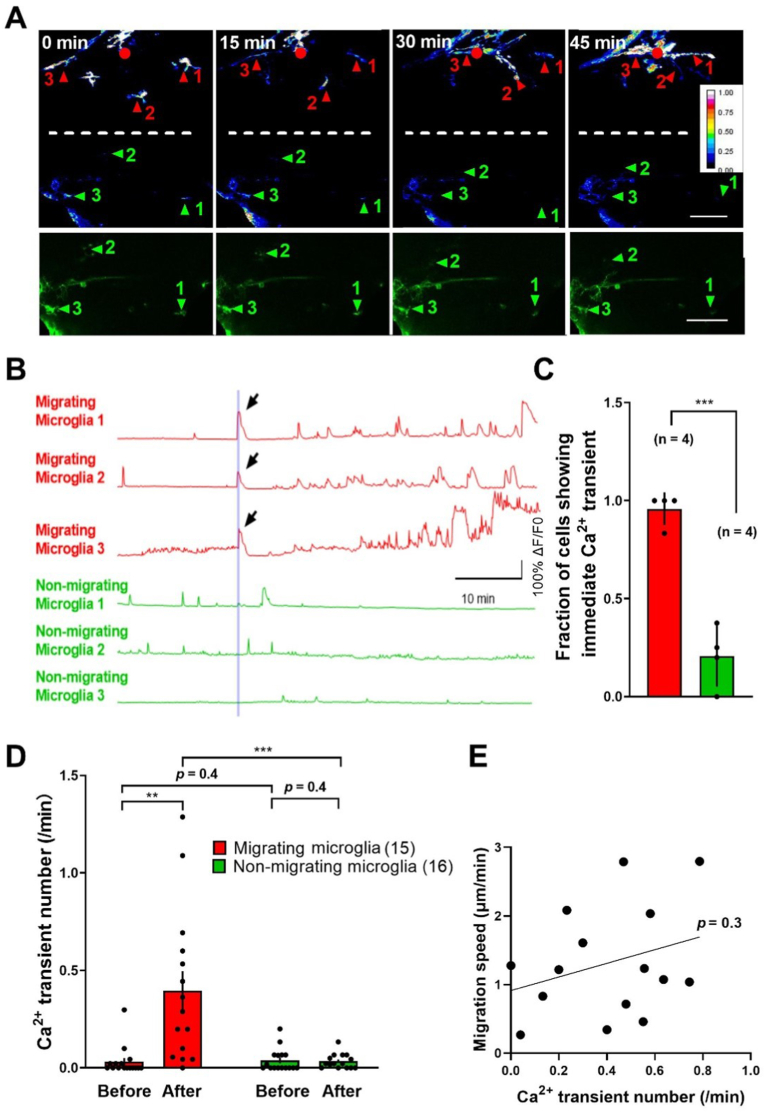
Then we examined the Ca2+ activity of microglia by monitoring changes in GCaMP5 signal (Fig. 2A). A strong Ca2+ transient activity was found in nearby microglia immediately after local brain injury and lasted for tens of seconds (arrow, Fig. 2B). And the follow-up Ca2+ burst activities were observed while those microglia were migrating towards the injury site (“migrating microglia”, red in Fig. 2A and B). Such Ca2+ activities were rarely found in those migrating microglia before injury. This type of Ca2+ activities were also rare in microglia located in the contralateral hemisphere to the injury site and did not exhibit directional migration after injury (“non-migrating microglia”, green in Fig. 2A and B).
Fig. 2.
Association between the Ca2+ activity and migration of activated microglia.
**p < 0.01, ***p < 0.001 (unpaired Student's t-test for (C) and for comparison between migrating and non-migrating microglia in (D), paired Student's t-test for comparison within migrating or non-migrating microglia in (D)). Error bars, SEM.
(A) Up, in vivo time-lapse two-photon images showing Ca2+ activities (in heatmaps) accompanying with microglia migration induced by laser-based local brain injury (red dot) at time 0. The data were obtained from the same larvae with that in Fig. 1B. Red numbers and arrowheads, microglia responsive to the injury; green numbers and arrowheads, microglia located in the contralateral hemisphere and non-responsive to the injury. Dashed white lines represent the midline between two hemispheres. Down, raw images showing the morphology of non-responsive microglia in the contralateral hemisphere. Scale bar, 50 μm
(B) Traces of Ca2+ activities of microglia numbered in (A). The vertical line indicates the onset of local brain injury. Arrow, immediate Ca2+ transient induced by the injury.
(C) Summary of the fraction of cells showing injury-induced immediate Ca2+ transient for the migrating (red) and non-migrating (green) microglia. Data were collected from 31 microglial cells in 4 zebrafish larvae, and each dot on the bar represents the data from an individual larva.
(D) Summary of injury-induced later Ca2+ transient number per minute before and after the local injury in migrating (red) and non-migrating (green) microglia. The number in the brackets represents the number of microglia examined, and each dot on the bar represents the data from an individual microglial cell. Data were collected from 31 microglial cells in 4 zebrafish larvae.
(E) Association between Ca2+ transient number per minute and migration speed before microglia stop moving on the injury site. The black linear line represents the simple linear regression slope (Y = 0.9932*X + 0.9130). Each dot on the graph represents the data from an individual microglial cell. Data were collected from 31 microglial cells in 4 zebrafish larvae. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To characterize the association between the Ca2+ activity and migration of microglia, we first analyzed the occurrence of Ca2+ transient immediately appeared after the injury in both the migrating and non-migrating microglia (Fig. 2C). We found that there is a significantly higher possibility that the migrating microglia have the immediate Ca2+ transients in comparison with the non-migrating microglia (p < 0.001, Fig. 2C). We then compared the follow-up Ca2+ burst activities in the migrating and non-migrating microglia before and after the local injury induction (Fig. 2D). Both the migrating and non-migrating microglia had comparable low Ca2+ activities before the local injury (p = 0.4). Whereas, after the injury, the number of Ca2+ transient events per minute increased markedly in the migrating microglia but not in the non-migrating microglia (p < 0.01 for migrating microglia, p = 0.4 for non-migrating microglia, Fig. 2D). The association between the number of calcium transient and migration speed shows an overall positive relationship but with no statistical significance (p = 0.3, Fig. 2E).
Taken together, these results indicate an association relationship between the migration and Ca2+ activity of activated microglia.
3.2. Ca2+activity was required for microglia migration towards the injury site
To further explore the role of Ca2+ activity in the migration of activated microglia, we examined the effect of Ca2+ activity suppression on microglia migration by local application of 2-APB, which is a general antagonist of IP3Rs. The 2-APB was loaded into the same micropipette used for local injury induction. Just as mentioned in Fig. 2, insertion of the micropipette induced elevated Ca2+ activity and directional migration of nearby microglia (“−10 min–0 min” data in Fig. 3A–D). After 10 min of injury-induced migration of microglia, 2-APB was puffed out until the end of imaging. As shown in Fig. 3A and B, after the local application of 2-APB, the level of Ca2+ activity in the migrating microglia decreased to the baseline. Importantly, the speed of its migration towards the injury site was reduced as well (Fig. 3A and B). In the control, extracellular solution (ES) was ejected out instead of 2-APB with the same pattern. However, after ES application, the microglial cell still exhibited Ca2+ activity and migrated to the injury site (Fig. 3C and D).
Fig. 3.
Requirement of Ca2+ activity for injury-induced migration of activated microglia.
*p < 0.05 (paired Student's t-test for comparison within each group before and after the puff, unpaired Student's t-test for comparison between groups of 2-APB and ES). Error bars, SEM.
(A) In vivo time-lapse two-photon images showing Ca2+ activity (in heatmaps) and migration of microglia evoked by micropipette-induced local brain injury (dashed orange lines) applied at time −10 min before the local puff of 2-APB (just after the time of 0 min). Scale bar, 10 μm
(B) Traces of both the Ca2+ activity (black) of the microglial cell and the distance (red) between the microglial cell body and micropipette tip for the case in (A). Gray area, application of 2-APB.
(C and D) Control case with puff of extracellular solution (ES) instead of 2-APB. Scale bar in (C), 10 μm
(E and F) Summary of changes in the Ca2+ activity (E) and migration speed (F) induced by puffing of ES or 2-APB. Each dot on the bar represents the data from an individual microglial cell examined. . (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
To quantify the effects of 2-APB, we calculated and compared the changes of Ca2+ activity and migration speed before and after the application of 2-APB or ES. Both the Ca2+ activity and migration speed significantly decreased after 2-APB application (p < 0.05 for both Ca2+ activity and migration speed) but not in the control group (p = 0.1 for Ca2+ activity and p = 0.3 for migration speed) (Fig. 3E and F). These results suggest that intracellular Ca2+ activity is required for injury-induced microglia directional migration.
4. Discussion
Under the physiological condition, microglia maintain at the resting state and locate dispersedly in the brain [1,2]. In response to local brain injury, nearby microglia will be activated and migrate through an ATP concentration gradient produced by injury-induced ATP release [[4], [5], [6]]. Our study reveals an increase of Ca2+ activity in migrating microglia as a necessary mechanism for injury-induced directional migration. A previous in vitro study reported that isolated mouse microglia displayed spontaneous Ca2+ activities [27]. Consistent with our finding, it was reported that Ca2+ is highly related to the migration of different cell types including endothelial tip cells [14], smooth muscle cells [15], macrophages [16], neural progenitors [17], and oligodendrocyte progenitors [18].
Intracellular Ca2+ stores play critical roles in various cell types in regulating Ca2+ levels in the cell. Previous in vitro studies showed that the intracellular Ca2+ store participates in Ca2+ activity in microglia and the inhibition of IP3Rs, a widely expressed channel for Ca2+ stores, can influence microglia behavior in the trans-well assay [28]. As an antagonist of IP3Rs, 2-APB was widely used to inhibit intracellular Ca2+ release from the store in various cell types including epithelial cells, platelets, and neutrophils [22]. Our results reveal a significant reduction of injury-induced follow-up Ca2+ bursts as well as migration speed after 2-APB application, suggesting the possibility of the involvement of IP3R-associated Ca2+ stores in the Ca2+ activity of migrating microglia and in mediating microglial cell migration. As it is reported that 2-APB may also affect multiple transient receptor potential (TRP) channels [29,30], future investigation is required to confirm the direct involvement of IP3Rs in the Ca2+ activity of activated microglia in vivo. In the present study, we did not exclude the participation of other types of glial cells in the microglial cell migration. It is possible that the 2-APB cause a decrease of intracellular Ca2+ concentration in the radial glial cells and thus a reduction of nitric oxide production [31], which could then affect microglial cell migration [32].
While our result suggested that the IP3R-induced endoplasmic reticulum (ER) Ca2+release is important for active microglial cell migration, there are other existing pathways that could be involved in the microglial cell migration shown in our study. Besides Ca2+, Gu et al. showed that the P2Y12 receptor, which is a possible upstream of the Ca2+ activity we observed, can also mediate K+ efflux, which may contribute to the electrophysiological activation of microglia [33]. As some of the potassium channels like kv1.3 and kir2.1 are found to contribute to microglial cell migration [34], the P2Y12-mediated K+ current might work as another downstream of P2 receptors to influence microglial cell migration. Other than the metabotropic P2 receptors, ionotropic P2X4 and P2X7 receptors can both respond to ATP and mediate Ca2+ influx [[35], [36], [37]]. In addition, the IP3R-mediated Ca2+ release can activate store-operated calcium entry (SOCE) by emptying the ER Ca2+ store [38,39], suggesting that IP3R can be even more important than just causing ER calcium release. These Ca2+ sources are shown to significantly impact intracellular Ca2+ activities and can modulate the microglial cell activation, migration, and inflammation response [36,38].
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We acknowledge Drs. Yu-Fan Wang, Ting-Ting Liu for helpful suggestions, Yu-Xi Li, Sha Li, Yu Qian, Tian-Lun Chen for supporting on imaging experiments, Le Sun for help in image analysis and figure plotting. This research was supported by Yang-Fan Program from Science and Technology Commission of Shanghai Municipality (14YF1406600). All authors contribute to this study. TD and XFD designed the study and wrote the manuscript. TD, XZ and RDZ performed the experiments. TD and XZ analyzed the data.
Contributor Information
Tian Du, Email: kristiandu.du@mail.utoronto.ca.
Xu-Fei Du, Email: xufeidu@ion.ac.cn.
References
- 1.Graeber M.B. Changing face of microglia. Science. 2010;330:783–788. doi: 10.1126/science.1190929. [DOI] [PubMed] [Google Scholar]
- 2.Hanisch U.K., Kettenmann H. Microglia: active sensor and versatile effector cells in the normal and pathologic brain. Nat. Neurosci. 2007;10:1387–1394. doi: 10.1038/nn1997. [DOI] [PubMed] [Google Scholar]
- 3.Ginhoux F., Greter M., Leboeuf M., Nandi S., See P., Gokhan S., et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science. 2010;330:841–845. doi: 10.1126/science.1194637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davalos D., Grutzendler J., Yang G., Kim J., Zuo Y., Jung S., et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8:752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 5.Haynes S.E., Hollopeter G., Yang G., Kurpius D., Dailey M.E., Gan W.B., et al. The P2Y12 receptor regulates microglial activation by extracellular nucleotides. Nat. Neurosci. 2006;9:1512–1519. doi: 10.1038/nn1805. [DOI] [PubMed] [Google Scholar]
- 6.Xu P., Xu Y., Hu B., Wang J., Pan R., Murugan M., et al. Extracellular ATP enhances radiation-induced brain injury through microglial activation and paracrine signaling via P2X7 receptor. Brain Behav. Immun. 2015;50:87–100. doi: 10.1016/j.bbi.2015.06.020. [DOI] [PubMed] [Google Scholar]
- 7.Paolicelli R.C., Bolasco G., Pagani F., Maggi L., Scianni M., Panzanelli P., et al. Synaptic pruning by microglia is necessary for normal brain development. Science. 2011;333:1456–1458. doi: 10.1126/science.1202529. [DOI] [PubMed] [Google Scholar]
- 8.Hughes A.N., Appel B. Microglia phagocytose myelin sheaths to modify developmental myelination. Nat. Neurosci. 2020;23:1055–1066. doi: 10.1038/s41593-020-0654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y., Du X.F., Liu C.S., Wen Z.L., Du J.L. Reciprocal regulation between resting microglial dynamics and neuronal activity in vivo. Dev. Cell. 2012;23:1189–1202. doi: 10.1016/j.devcel.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 10.Parkhurst C.N., Yang G., Ninan I., Savas J.N., Yates J.R., Lafaille J.J., et al. Microglia promote learning-dependent synapse formation through brain-derived neurotrophic factor. Cell. 2013;155:1596–1609. doi: 10.1016/j.cell.2013.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang C., Yue H., Hu Z., Shen Y., Ma J., Li J., et al. Microglia mediate forgetting via complement-dependent synaptic elimination. Science. 2020;367:688–694. doi: 10.1126/science.aaz2288. [DOI] [PubMed] [Google Scholar]
- 12.Marín-Teva J.L., Dusart I., Colin C., Gervais A., Rooijen N.V., Mallat M. Microglia promote the death of developing purkinje cells. Neuron. 2004;41:535–547. doi: 10.1016/s0896-6273(04)00069-8. [DOI] [PubMed] [Google Scholar]
- 13.Xu J., Wang T., Wu Y., Jin W., Wen Z. Microglia colonization of developing Zebrafish midbrain is promoted by apoptotic neuron and lysophosphatidylcholine. Dev. Cell. 2016;38:214–222. doi: 10.1016/j.devcel.2016.06.018. [DOI] [PubMed] [Google Scholar]
- 14.Liu T.T., Du X.F., Zhang B.B., Zi H.X., Yan Y., Yin J.A., et al. Piezo1-Mediated Ca2+ activities regulate brain vascular pathfinding during development. Neuron. 2020;108:180–192. doi: 10.1016/j.neuron.2020.07.025. [DOI] [PubMed] [Google Scholar]
- 15.Pfleiderer P.J., Lu K.K., Crow M.T., Keller R.S., Singer H.A. Modulation of vascular smooth muscle cell migration by calcium/calmodulin-dependent protein kinase II-δ2. Am. J. Physiol. Cell Physiol. 2004;286:1238–1245. doi: 10.1152/ajpcell.00536.2003. [DOI] [PubMed] [Google Scholar]
- 16.Damann N., Owsianik G., Li S., Poll C., Nilius B. The calcium-conducting ion channel transient receptor potential canonical 6 is involved in macrophage inflammatory protein-2-induced migration of mouse neutrophils. Acta Physiol. 2009;195:3–11. doi: 10.1111/j.1748-1716.2008.01918.x. [DOI] [PubMed] [Google Scholar]
- 17.Uhlén P., Fritz N., Smedler E., Malmersjö S., Kanatani S. Calcium signaling in neocortical development. Dev. Neurobiol. 2015;75:360–368. doi: 10.1002/dneu.22273. [DOI] [PubMed] [Google Scholar]
- 18.Piller M., Werkman I.L., Brown E.A., Latimer A.J., Kucenas S. Glutamate signaling via the AMPAR subunit gluR4 regulates oligodendrocyte progenitor cell migration in the developing spinal cord. J. Neurosci. 2021;41:5353–5371. doi: 10.1523/JNEUROSCI.2562-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brawek B., Garaschuk O. Microglial calcium signaling in the adult, aged and diseased brain. Cell Calcium. 2013;53:159–169. doi: 10.1016/j.ceca.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 20.Hoffmann A., Kann O., Ohlemeyer C., Hanisch U.K., Kettenmann H. Elevation of basal intracellular calcium as a central element in the activation of brain macrophages (microglia): suppression of receptor-evoked calcium signaling and control of release function. J. Neurosci. 2003;23:4410–4419. doi: 10.1523/JNEUROSCI.23-11-04410.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friedrich R.W., Wanner A.A. Dense circuit reconstruction to understand neuronal computation: focus on zebrafish. Annu. Rev. Neurosci. 2021;44:275–293. doi: 10.1146/annurev-neuro-110220-013050. [DOI] [PubMed] [Google Scholar]
- 22.Maruyama T., Kanaji T., Nakade S., Kanno T., Mikoshiba K. 2APB, 2- aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 1997;122 doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 23.Li L., Yan B., Shi Y.Q., Zhang W.Q., Wen Z.L. Live imaging reveals differing roles of macrophages and neutrophils during zebrafish tail fin regeneration. J. Biol. Chem. 2012;287:25353–25360. doi: 10.1074/jbc.M112.349126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu J., Pan L., Shang C., Lu B., Wu R., Feng Y., et al. A highly sensitive and selective nanosensor for near-infrared potassium imaging. Sci. Adv. 2020;6:1–11. doi: 10.1126/sciadv.aax9757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Du X.F., Xu B., Zhang Y., Chen M.J., Du J.L. A transgenic zebrafish model for in vivo long-term imaging of retinotectal synaptogenesis. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-32409-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang B.B., Yao Y.Y., Zhang H.F., Kawakami K., Du J.L. Left Habenula mediates light-preference behavior in Zebrafish via an asymmetrical visual pathway. Neuron. 2017;93:914–928. doi: 10.1016/j.neuron.2017.01.011. e4. [DOI] [PubMed] [Google Scholar]
- 27.Korvers L., Costa A.A., Mersch M., Matyash V., Kettenmann H., Semtner M. Spontaneous Ca2+ transients in mouse microglia. Cell Calcium. 2016;60:396–406. doi: 10.1016/j.ceca.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 28.Meng X.L., Chen C.L., Liu Y.Y., Su S.J., Gou J.M., Huan F.N., et al. Selenoprotein SELENOK enhances the migration and phagocytosis of microglial cells by increasing the cytosolic free Ca2+ level resulted from the up-regulation of IP 3 R. Nat. Neurosci. 2019;406:38–49. doi: 10.1016/j.neuroscience.2019.02.029. [DOI] [PubMed] [Google Scholar]
- 29.Delmas P., Wanaverbecq N., Abogadie F.C., Mistry M., Brown D.A. Signaling microdomains define the specificity of receptor-mediated InsP(3) pathways in neurons. Neuron. 2002;34:209–220. doi: 10.1016/s0896-6273(02)00641-4. [DOI] [PubMed] [Google Scholar]
- 30.Xu S.Z., Zeng F., Boulay G., Grimm C., Harteneck C., Beech D.J. Block of TRPC5 channels by 2-aminoethoxydiphenyl borate: a differential, extracellular and voltage-dependent effect. Br. J. Pharmacol. 2005;145:405–414. doi: 10.1038/sj.bjp.0706197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Feinstein D.L., Galea E., Cermak J., Chugh P., Lyandvert L., Reis D.J. Nitric oxide synthase expression in glial cells: suppression by tyrosine kinase inhibitors. J. Neurochem. 1994;62:811–814. doi: 10.1046/j.1471-4159.1994.62020811.x. [DOI] [PubMed] [Google Scholar]
- 32.Dibaj P., Nadrigny F., Steffens H., Scheller A., Hirrlinger J., Schomburg E.D. NO mediates microglial response to acute spinal cord injury under ATP control in vivo. Glia. 2010;58:1133–1144. doi: 10.1002/glia.20993. [DOI] [PubMed] [Google Scholar]
- 33.Gu N., Eyo U.B., Murugan M., Peng J., Matta S., Dong H., et al. Microglial P2Y12 receptors regulate microglial activation and surveillance during neuropathic pain. Brain Behav. Immun. 2016;55:82–92. doi: 10.1016/j.bbi.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anton R., Ghenghea M., Ristoiu V., Gattlen C., Suter M.-R., Cojocaru P.A., et al. Potassium channels KV1.3 and kir2.1 but not KV1.5 contribute to BV2 cell line and primary microglial migration. Int. J. Mol. Sci. 2021;22:2081. doi: 10.3390/ijms22042081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bernier L.-P., Ase A.R., Chevallier S., Blais D., Zhao Q., Boue-Grabot E., et al. Phosphoinositides regulate P2X4 ATP-gated channels through direct interactions. J. Neurosci. 2008;28:12938–12945. doi: 10.1523/jneurosci.3038-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohsawa K., Irino Y., Nakamura Y., Akazawa C., Inoue K., Kohsaka S. Involvement of P2X4and P2Y12receptors in ATP-induced microglial chemotaxis. Glia. 2007;55:604–616. doi: 10.1002/glia.20489. [DOI] [PubMed] [Google Scholar]
- 37.Janks L., Sharma C.V., Egan T.M. A central role for P2X7 receptors in human microglia. J. Neuroinflammation. 2018;15 doi: 10.1186/s12974-018-1353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gilbert D.F., Stebbing M.J., Kuenzel K., Murphy R.M., Zacharewicz E., Buttgereit A., et al. Store-operated Ca2+ entry (SOCE) and purinergic receptor-mediated Ca2+ homeostasis in murine BV2 microglia cells: early cellular responses to ATP-mediated microglia activation. Front. Mol. Neurosci. 2016;9 doi: 10.3389/fnmol.2016.00111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sampieri A., Santoyo K., Asanov A., Vaca L. Association of the IP3R to STIM1 provides a reduced intraluminal calcium microenvironment, resulting in enhanced store-operated calcium entry. Sci. Rep. 2018;8 doi: 10.1038/s41598-018-31621-0. [DOI] [PMC free article] [PubMed] [Google Scholar]