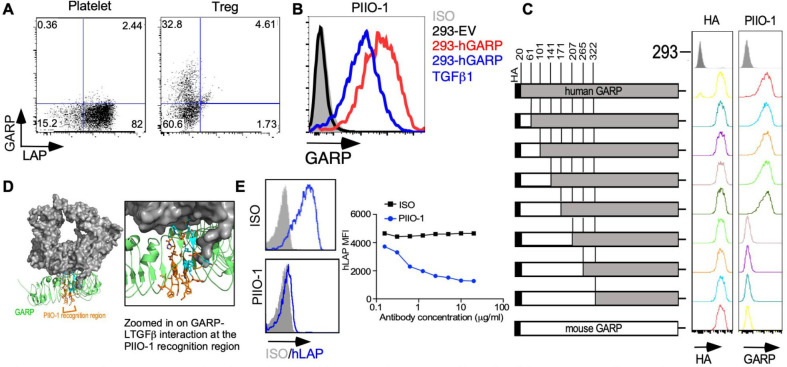
Figure 2.
In vitro characterization of anti-GARP antibody PIIO-1. (A) GARP expression on human regulatory T cells and platelets was evaluated by flow cytometry after staining with PIIO-1 at 10 µg/mL. (B) 293 FT cell line was transfected with empty vector (EV), human GARP (hGARP)-expressing vector only or co-transfected with hGARP and latent TGFβ1 expression vectors. GARP expression on indicated cell line was detected by flow cytometry after staining with PIIO-1 at 10 µg/mL. (C) Human GARP sequence was replaced by murine GARP according to the schematic diagram to generate the chimeric constructs of human and murine GARP that were tagged with HA (hemagglutinin) epitope. Transfection efficiency was determined using anti-HA antibody. All constructs were transfected into 293 FT cells. (D) Crystal structure of the GARP (green)-LTGFβ (gray) complex (PDB DOI: 10.2210/pdb6GFF/pdb). The region of PIIO-1 recognition is orange and the residues interacting with LTGFβ are cyan. LTGFβ occludes approximately 30% of the potential antibody binding site and may sterically or allosterically restrict access of the antibody to GARP in the LTGFβ-complexed state. Modeling was carried out using Pymol. (E) Jurkat cell line, made to overexpress hGARP, was incubated with LTGFβ1 along with mIgG1 or PIIO-1 at indicated concentration for 30 min at 37℃. Human LAP expression was detected by flow cytometry. All data are representative of 2–6 independent experiments. GARP, Glycoprotein-A repetitions predominant; LAP, latency-associated peptide.