Abstract
Background
Rheumatoid arthritis has been associated with severe COVID-19, but few studies have investigated how phenotypes of rheumatoid arthritis affect these associations. We aimed to investigate the associations between rheumatoid arthritis and phenotypes of interstitial lung disease, serostatus, and bone erosions with COVID-19 severity.
Methods
We did a retrospective, comparative, multicentre cohort study at two large health-care systems (Mayo Clinic [19 hospitals and affiliated outpatient centres] and Mass General Brigham [14 hospitals and affiliated outpatient centres]) in the USA. Consecutive patients with rheumatoid arthritis meeting the 2010 American College of Rheumatology/European League Against Rheumatism classification criteria and who had COVID-19 between March 1, 2020, and June 6, 2021, were matched 1:5 on age, sex, and calendar date with patients without rheumatoid arthritis (comparators). Data were received from electronic health records from Mayo Clinic and Mass General Brigham. We examined subgroups of patients with rheumatoid arthritis by phenotypic features: rheumatoid arthritis-associated interstitial lung disease, seropositivity (for anti-cyclic citrullinated peptide, rheumatoid factor, or both), and bone erosions. Severe COVID-19 was a composite of hospitalisation or death. We used Cox regression to estimate hazard ratios (HR) for severe COVID-19, comparing rheumatoid arthritis and subgroups to the comparator group.
Findings
We identified 582 patients with rheumatoid arthritis and 2875 matched comparators, all of whom had COVID-19 within the study dates. The mean age of those with rheumatoid arthritis was 62 [SD 14] years, 421 (72%) of 582 were women and 161 (28%) were men, 457 (79%) were White, 65 (11%) were Hispanic or Latino, and 41 (7%) were Black. Among patients with rheumatoid arthritis, 50 (9%) of 582 had interstitial lung disease, 388 (68%) of 568 were seropositive, and 159 (27%) of 582 had bone erosions. Severe COVID-19 occurred in 126 (22%) of 582 patients with rheumatoid arthritis versus 363 (13%) 2875 in the comparator group. Patients with rheumatoid arthritis had an HR of 1·75 (95% CI 1·45–2·10) for severe COVID-19 versus the comparator group. Patients with rheumatoid arthritis-associated interstitial lung disease had an HR of 2·50 (1·66–3·77) versus the comparator group for severe COVID-19. The risk for severe COVID-19 was also higher in patients with rheumatoid arthritis who were seropositive (HR 1·97 [95% CI 1·58–2·46]) or had erosive disease (1·93 [1·41–2·63]) than for those in the comparator group.
Interpretation
Patients with rheumatoid arthritis have an increased risk of severe COVID-19 across phenotypic subgroups, especially among patients with interstitial lung disease. These findings suggest that rheumatoid arthritis with interstitial lung disease, or its treatment, might be a substantial contributor to severe COVID-19 outcomes for patients with rheumatoid arthritis.
Funding
None.
Introduction
During the ongoing COVID-19 pandemic, clinically vulnerable populations, including those with rheumatic diseases, have faced uncertainty regarding the potential increased risk of SARS-CoV-2 infection and adverse COVID-19 outcomes.1 This uncertainty has typically been addressed in general cohorts of patients with autoimmune disease in both international registry and multicentre studies, with some studies reporting links between systemic autoimmune disease and risk of severe COVID-19 outcomes,2, 3, 4, 5 including death. However, some studies have found no association.6 Combining heterogeneous rheumatic diseases with diverse manifestations and treatments into a single group might obscure important factors, such as specific disease phenotypes that might be responsible for poor outcomes in these populations.
Studies suggest that patients with rheumatoid arthritis are at higher risk of SARS-CoV-2 infection and severe COVID-19 outcomes.2, 3, 4, 5, 6, 7, 8, 9 However, little is known regarding specific phenotypic features of rheumatoid arthritis commonly used to inform management, such as interstitial lung disease, serostatus, and bone erosions, and their effect on COVID-19 outcomes. Previous studies investigating the association of rheumatoid arthritis with COVID-19 have been limited by including a population with mostly male patients,7 absence of details regarding erosive disease or serostatus,3, 8, 9 and risk of diagnosis misclassification;3, 7, 9 only one study examined rheumatoid arthritis serostatus.7 Chronic lung disease has been associated with an increased risk of severe COVID-19,10, 11, 12 and a single case-control study found an increased risk of death for patients with interstitial lung disease and COVID-19;13 no studies have examined whether patients with rheumatoid arthritis-associated interstitial lung disease, or other subgroups of patients with rheumatoid arthritis, might be particularly vulnerable to COVID-19.
Research in context.
Evidence before this study
We searched PubMed for articles published from database inception to April 15, 2022, using the terms: ((“rheumatoid arthritis”) AND (COVID-19 OR SARS-CoV-2) AND (“outcome*”)) NOT ((review) OR (editorial) OR (“case report”)). We found 107 articles. The most relevant reports included a study using the Optum dataset that described an increased risk of hospitalisation, but not of mortality, from COVID-19 after adjustment for comorbidities in patients with rheumatoid arthritis, but the study used administrative data to define rheumatoid arthritis and had no phenotypic data. A rheumatoid arthritis cohort study from the US Veterans Affairs system that included mostly male participants and did not examine rheumatoid arthritis and interstitial lung disease or bone erosions reported an increased risk of severe COVID-19 compared with the matched controls; this risk was independent of serostatus. A Swedish nationwide study found that patients with rheumatoid arthritis had a higher risk of hospitalisation and death due to COVID-19 compared with the general population, but the study did not investigate rheumatoid arthritis phenotypes. Finally, a large US research network cohort study that found an increased risk of severe COVID-19 outcomes before propensity score matching to general population comparators, which was mitigated after matching. There was a paucity of evidence regarding the effect that different phenotypes of rheumatoid arthritis (eg, interstitial lung disease, serostatus, or erosive disease) might have on COVID-19 outcomes.
Added value of this study
We did a study involving more than 30 sites in the USA to describe the risk of severe COVID-19 outcomes associated with rheumatoid arthritis overall and stratified according to the presence of interstitial lung disease, serostatus, and bone erosions in comparison with matched patients without rheumatoid arthritis with COVID-19. We found that rheumatoid arthritis-associated interstitial lung disease was particularly associated with increased risk of severe COVID-19.
Implications of all the available evidence
Our findings suggest that one driver of the increased risk of severe COVID-19 in patients with rheumatoid arthritis is the presence of interstitial lung disease or its treatment. The results of this study inform individualised COVID-19 risk stratification for patients with rheumatoid arthritis.
Our objective was to compare severe COVID-19 outcomes for patients with rheumatoid arthritis with those of patients without rheumatoid arthritis and to examine these associations according to key phenotypic features of rheumatoid arthritis.
Methods
Study design and participants
We did a retrospective, comparative, multicentre cohort study at Mayo Clinic (19 hospitals and affiliated outpatient centres in Minnesota, Florida, Arizona, and Wisconsin) and Mass General Brigham (14 hospitals and affiliated outpatient centres in Massachusetts) in the USA. We identified patients with rheumatoid arthritis who had COVID-19 from March 1, 2020 to June 6, 2021. We followed up with each patient for severe outcomes occurring within 90 days of initial positive SARS-CoV-2 test. Data collection was completed on October 1, 2021. We included patients meeting the 2010 American College of Rheumatology/European League Against Rheumatism (ACR/EULAR) rheumatoid arthritis classification criteria and who had COVID-19.
We matched each patient with confirmed rheumatoid arthritis and COVID-19 (cases) to five patients with COVID-19 and without recorded rheumatoid arthritis (comparators) from the same health-care system (ie, Mayo Clinic or Mass General Brigham). We used up to a 1:5 ratio because the precision of the effect estimate increases as the sample size increases but little is gained in precision by increasing this ratio beyond 1:5. The pool of eligible comparators had never received a billing or diagnosis code for rheumatoid arthritis and had a positive SARS-CoV-2 test. The index date was the first date of a positive SARS-CoV-2 test. Matching factors were institution (Mayo Clinic or Mass General Brigham), age at index date (within 5 years), sex, and calendar date of first positive SARS-CoV-2 test (within 5 days of index date at Mayo Clinic and within 7 days at Mass General Brigham). We matched by calendar date to account for changes in testing availability, hospital capacity, treatment, prevention strategies (eg, vaccination), and virus epidemiology (eg, surges and variants) over time. We have used similar methods in previous studies.14, 15
The study was approved by the institutional review boards of Mayo Clinic (20–003167) and Mass General Brigham (2020P000833). Patient consent was not required for this retrospective study.
Procedures
For Mayo Clinic data, we queried electronic health records to identify all patients with International Classification of Diseases 10th Revision (ICD-10) codes for rheumatoid arthritis who were tested for rheumatoid factor or anti-cyclic citrullinated peptide (anti-CCP) antibody and had any positive SARS-CoV-2 PCR test. For Mass General Brigham data, we queried electronic health records to identify all patients with a positive SARS-CoV-2 test (PCR or rapid antigen detection test) who had any billing code for rheumatoid arthritis, as detailed elsewhere.14, 15, 16 For both institutions, we (GF-P, ELG, MOV-A, SV, NJP, CC, RH, GCM, MAD, LM, KMMV, EK, GQ, ZSW, AD-G, and JAS) manually reviewed medical records and included all consecutive patients meeting the 2010 ACR/EULAR rheumatoid arthritis classification criteria17 and who had COVID-19 between March 1, 2020, and June 6, 2021.
We queried electronic health records to obtain age, sex, race, ethnicity, body-mass index (BMI), smoking status (never, former, or current), and Charlson comorbidity index18 and its components (from billing codes within 1 year before the index date) for patients with rheumatoid arthritis and comparators.
We reviewed medical records of patients with rheumatoid arthritis in detail to obtain additional data from on or before the index date. The criteria used to identify the presence of rheumatoid arthritis-associated interstitial lung disease were the clinical diagnosis of interstitial lung disease and supportive chest radiology,19 pulmonary function testing, or lung pathology data; rheumatoid factor and anti-CCP serostatus according to assay normal ranges that were clinically obtained; and the presence of bone erosions on imaging. We also collected details regarding rheumatoid arthritis duration and use of disease-modifying anti-rheumatic drugs (DMARDs) and glucocorticoids at the time of SARS-CoV-2 onset. Medical record review was used to supplement data on serostatus if missing. Otherwise, statistical models had complete data on covariates.
Outcome
Our primary outcome was a composite for severe COVID-19 (hospitalisation or death) after COVID-19 diagnosis among patients with rheumatoid arthritis overall and subgroups compared with the matched population without rheumatoid arthritis. For data collected from Mayo Clinic, hospitalisation or death due to COVID-19 was ascertained by medical record review based on discharge diagnosis. For data collected from Mass General Brigham, hospitalisation was attributed to COVID-19 if it occurred within 14 days of initial positive test, and death during follow-up was attributed to COVID-19 if it occurred within 30 days of initial positive test, as done in our previous studies.14, 15, 20 Our secondary outcomes were hospitalisation and death (separately), and requirement for mechanical ventilation. In a secondary analysis, we stratified by rheumatoid factor and anti-CCP status separately. We did a sensitivity analysis for patients with rheumatoid arthritis and comparators before the vaccines were available in the USA (before Dec 15, 2020).
Statistical analysis
We used descriptive statistics to summarise the data. We calculated follow-up time from the index date (date of earliest positive SARS-CoV-2 test) to the hospitalisation date, death date, or 90 days after the index date (whichever came first), and estimated the rate (95% CI) of each outcome during the study period. We then estimated the rate difference (95% CI) comparing patients with rheumatoid arthritis and the phenotypic subgroups (rheumatoid arthritis-associated interstitial lung disease, rheumatoid factor and/or anti-CCP seropositivity, and erosive disease) with matched comparators. We used conditional unadjusted and adjusted multivariable Cox regression models to estimate the hazard ratio (HR; 95% CI) of patients with rheumatoid arthritis, and subgroups, for COVID-19 outcomes compared with matched comparators. The main adjusted model included age, sex, race, and smoking status. We used an additional model that also adjusted for BMI and the Charlson comorbidity index, recognising that these data might mediate the association between rheumatoid arthritis and COVID-19 severity. We assessed the p values for heterogeneity using an interaction term for the association of rheumatoid arthritis with interstitial lung disease versus other phenotypes with the risk of severe outcomes to investigate whether there were differences in the risk of severe outcomes across phenotypes of rheumatoid arthritis.
We also assessed the risk of a severe outcome in each of eight phenotypes of rheumatoid arthritis versus comparators on the basis of the presence of interstitial lung disease (yes or no), seropositivity (yes or no), and erosive disease (yes or no). In each of the eight phenotypic combinations, we determined the risk of severe COVID-19 outcome in patients with rheumatoid arthritis and their matched comparators and calculated the rate difference between both groups. Our primary exposure of interest was patients with rheumatoid arthritis versus matched comparators; other exposures of interest, including rheumatoid arthritis phenotypic subgroups and their comparators, should be considered exploratory and were not adjusted for multiple testing. We considered a p value of <0·05 as statistically significant for all analyses. We did analyses using SAS (version 9.4).
Role of the funding source
There was no funding source for this study.
Results
We included 582 patients with rheumatoid arthritis and 2875 patients without rheumatoid arthritis who were comparators, all with COVID-19 (table 1 ). Patients with rheumatoid arthritis and comparators were well matched with regard to age (mean age 62 [SD 14] years for patients with rheumatoid arthritis vs 61 [14] years for comparators), and sex (421 [72%] of 582 patients with rheumatoid arthritis and 2081 [72%] of 2875 comparators were women). 457 [79%] of 582 patients with rheumatoid arthritis and 2294 [80%] of 2875 comparators were White. Among patients with rheumatoid arthritis, median duration of rheumatoid arthritis was 8 (IQR 4–15) years, 50 (9%) of 582 had rheumatoid arthritis and interstitial lung disease, 388 (68%) of 568 were seropositive (302 [54%] of 557 were positive for rheumatoid factor and 312 [58%] of 540 were positive for anti-CCP), 159 (27%) had bone erosions, 163 (28%) of 575 were using glucocorticoids, and 457 (79%) of 582 were using DMARDs at the time of SARS-CoV-2 infection. 366 [63%] of 582 patients with rheumatoid arthritis and 1808 [63%] of 2875 comparators had COVID-19 before vaccines against SARS-CoV-2 were available, and only 21 (4%) of 582 patients with rheumatoid arthritis had breakthrough infections. We did not have data on breakthrough infections for the comparator group. The demographics and disease-specific characteristics stratified by rheumatoid arthritis subgroups are in table 2 .
Table 1.
Demographic and clinical characteristics of patients with rheumatoid arthritis and age, sex, and calendar-matched patients without rheumatoid arthritis (comparator group) at first positive SARS-CoV-2 test
| Patients with rheumatoid arthritis (n=582) | Patients without rheumatoid arthritis (n=2875) | p value | ||
|---|---|---|---|---|
| Mean age (SD), years | 62 (14) | 61 (14) | 0·53 | |
| Sex | .. | .. | 0·98 | |
| Female | 421 (72%) | 2081 (72%) | .. | |
| Male | 161 (28%) | 794 (28%) | .. | |
| Race | .. | .. | 0·21 | |
| White | 457 (79%) | 2294 (80%) | .. | |
| Black | 41 (7%) | 221 (8%) | .. | |
| Asian | 15 (3%) | 67 (2%) | .. | |
| Other | 48 (8%) | 171 (6%) | .. | |
| Unknown | 21 (4%) | 122 (4%) | .. | |
| Hispanic or Latin Ethnicity* | 65 (11%) | 259/2669 (10%) | 0·29 | |
| Mean body-mass index* (SD), kg/m2 | 29·8 (7·5) | 30·7 (7·4) | 0·010 | |
| Smoking status | .. | .. | 0·027 | |
| Never | 307 (53%) | 1615 (56%) | .. | |
| Former | 230 (40%) | 934 (32%) | .. | |
| Current | 42 (7%) | 192 (7%) | .. | |
| Unknown | 3 (1%) | 134 (5%) | .. | |
| Median Charlson comorbidity index* (IQR) | 3 (1–4) | 2 (0–3) | <0·0001 | |
| Median rheumatoid arthritis duration (IQR), years | 8 (4–15) | .. | .. | |
| Seropositivity*† | 388 (68%) of 568 | .. | .. | |
| Rheumatoid factor | 302 (54%) of 557 | .. | .. | |
| Anti-CCP | 312 (58%) of 540 | .. | .. | |
| Bone erosions | 159 (27%) | .. | .. | |
| Interstitial lung disease, n (%) | 50 (9%) | .. | .. | |
| Rheumatoid arthritis medications | ||||
| Any DMARDs | 457 (79%) | .. | .. | |
| Conventional synthetic DMARD | 362 (62%) | .. | .. | |
| Methotrexate | 219 (38%) | .. | .. | |
| Leflunomide | 45 (8%) | .. | .. | |
| Sulfasalazine | 30 (5%) | .. | .. | |
| Hydroxychloroquine | 129 (22%) | .. | .. | |
| Biologic or targeted-synthetic DMARD | 194 (33%) | .. | .. | |
| Tumour necrosis factor inhibitors | 112 (19%) | .. | .. | |
| Janus kinase inhibitors | 32 (5%) | .. | .. | |
| Rituximab | 28 (5%) | .. | .. | |
| Abatacept | 17 (3%) | .. | .. | |
| Interleukin-6 inhibitors | 7 (1%) | .. | .. | |
| Mycophenolate mofetil | 12 (2%) | .. | .. | |
| Glucocorticoid use* | 163 (28%) of 575 | .. | .. | |
| Median glucocorticoid dose (IQR), mg/day | 5 (5–10) | .. | .. | |
| Glucocorticoid use without DMARDs | 25 (4%) of 575 | .. | .. | |
Data are n (%), unless specified. Anti-CCP=anti-cyclic citrullinated peptide antibody. DMARD=disease-modifying anti-rheumatic drug.
The calculation excludes missing or unknown data; for Hispanic or Latin ethnicity there were data missing for 206 (7%) patients in the comparator group; for body-mass index there were eight (1%) patients with rheumatoid arthritis with missing data and 254 (9%) with missing data in the comparator group; for Charlson comorbidity index there were nine (2%) patients with rheumatoid arthritis with missing data and 48 (2%) in the comparator group; for serostatus there were 14 (2%) patients with rheumatoid arthritis with missing data; and for glucocorticoid use there were seven (1%) patients with rheumatoid arthritis with missing data.
Positivity for either rheumatoid factor or anti-CCP.
Table 2.
Demographic and clinical characteristics among patients with rheumatoid arthritis according to rheumatoid arthritis phenotypes at time of SARS-CoV-2 infection
|
Interstitial lung disease |
Serostatus* |
Bone erosions |
|||||
|---|---|---|---|---|---|---|---|
| Positive (n=50) | Negative (n=532) | Positive (n=388) | Negative (n=180) | Positive (n=159) | Negative (n=423) | ||
| Mean age (SD), years | 61 (14) | 71 (11) | 62 (14) | 60 (14) | 65 (14) | 60 (15) | |
| Sex | |||||||
| Female | 25 (50%) | 396 (74%) | 275 (71%) | 134 (74%) | 123 (77%) | 298 (70%) | |
| Male | 25 (50%) | 136 (26%) | 113 (29%) | 46 (26%) | 36 (23%) | 125 (30%) | |
| Race | |||||||
| White | 34 (68%) | 423 (80%) | 299 (77%) | 149 (83%) | 119 (75%) | 338 (80%) | |
| Black | 8 (16%) | 33 (6%) | 28 (7%) | 12 (7%) | 12 (8%) | 29 (7%) | |
| Asian | 0 (0%) | 15 (3%) | 11 (3%) | 2 (1%) | 4 (3%) | 11 (3%) | |
| Other | 5 (10%) | 43 (8%) | 38 (10%) | 10 (6%) | 15 (9%) | 33 (8%) | |
| Unknown | 3 (6%) | 18 (3%) | 12 (3%) | 7 (4%) | 9 (6%) | 12 (3%) | |
| Hispanic or Latin Ethnicity | 9 (18%) | 56 (11%) | 48 (12%) | 16 (9%) | 24 (15%) | 41 (10%) | |
| Mean body-mass index† (SD), kg/m2 | 29·9 (7·7) | 28·8 (5·0) | 29·3 (6·5) | 31·2 (9·2) | 28·7 (6·1) | 30·2 (8·0) | |
| Missing | 0 (0%) | 8 (2%) | 6 (2%) | 1 (1%) | 1 (1%) | 7 (2%) | |
| Smoking status | |||||||
| Never | 17 (34%) | 290 (55%) | 198 (51%) | 101 (56%) | 84 (53%) | 223 (53%) | |
| Former | 32 (64%) | 198 (37%) | 159 (41%) | 65 (36%) | 61 (38%) | 169 (40%) | |
| Current | 1 (2%) | 41 (8%) | 30 (8%) | 12 (7%) | 14 (9%) | 28 (7%) | |
| Unknown | 0 (0%) | 3 (1%) | 1 (<1%) | 2 (1%) | 0 (0%) | 3 (1%) | |
| Charlson comorbidity index†, median (IQR) | 3 (1–4) | 4 (2–6) | 3 (1·5–5) | 2 (1–4) | 3 (2–5) | 3 (1–4) | |
| Missing | 1 (2%) | 8 (2%) | 8 (2%) | 1 (1%) | 1 (1%) | 8 (2%) | |
| COVID-19 vaccine uptake | 1 (2%) | 20 (4%) | 13 (3%) | 8 (4%) | 3 (2%) | 18 (4%) | |
| Mean rheumatoid arthritis duration (SD), years | 8 (4–14) | 10 (5–18) | 9 (4–16) | 7 (3–12) | 11·5 (6–20) | 7 (3–13) | |
| Seropositivity* | 42 (84%) | 346 (65%) | 388 (100%) | .. | 119 (75%) | 269 (64%) | |
| Rheumatoid factor | 33 (66%) | 269 (51%) | 302 (78%) | .. | 100 (63%) | 202 (48%) | |
| Anti-CCP | 32 (64%) | 280 (53%) | 312 (80%) | .. | 100 (63%) | 212 (50%) | |
| Bone erosions | 14 (28%) | 145 (27%) | 119 (31%) | 37 (21%) | 159 (100%) | .. | |
| Interstitial lung disease | 50 (100%) | .. | 42 (11%) | 8 (4%) | 14 (9%) | 36 (9%) | |
| Classification of ILD | |||||||
| Definite | 25 (50%) | .. | 21 (5%) | 4 (2%) | 8 (5%) | 17 (4%) | |
| Probable | 9 (18%) | .. | 8 (2%) | 1 (1%) | 2 (1%) | 7 (2%) | |
| Possible | 16 (32%) | .. | 13 (3%) | 3 (2%) | 4 (3%) | 12 (3%) | |
| Severity of ILD | |||||||
| Subclinical | 15 (30%) | .. | 12 (3%) | 3 (2%) | 1 (1%) | 14 (3%) | |
| Mild | 18 (36%) | .. | 16 (4%) | 2 (1%) | 8 (5%) | 10 (2%) | |
| Moderate | 10 (20%) | .. | 8 (2%) | 2 (1%) | 5 (3%) | 5 (1%) | |
| Severe | 6 (12%) | .. | 5 (1%) | 1 (1%) | 0 (0%) | 6 (1%) | |
| Missing | 1 (2%) | .. | 1 (<1%) | 0 | 0 | 1 (<1%) | |
| Rheumatoid arthritis medications | |||||||
| Any DMARD | 42 (84%) | 415 (78%) | 311 (80%) | 136 (76%) | 135 (85%) | 322 (76%) | |
| Conventional synthetic DMARD | 34 (68%) | 328 (62%) | 246 (63%) | 108 (60%) | 103 (65%) | 259 (61%) | |
| Methotrexate | 14 (28%) | 205 (39%) | 152 (39%) | 60 (33%) | 70 (44%) | 149 (35%) | |
| Leflunomide | 5 (10%) | 40 (8%) | 27 (7%) | 18 (10%) | 15 (9%) | 30 (7%) | |
| Sulfasalazine | 2 (4%) | 28 (5%) | 19 (5%) | 9 (5%) | 6 (4%) | 24 (6%) | |
| Hydroxychloroquine | 10 (20%) | 119 (22%) | 85 (22%) | 42 (23%) | 29 (18%) | 100 (24%) | |
| Biologic or targeted-synthetic DMARD | 19 (38%) | 175 (33%) | 135 (35%) | 56 (31%) | 62 (39%) | 132 (31%) | |
| Tumour necrosis factor inhibitors | 8 (16%) | 104 (20%) | 78 (20%) | 31 (17%) | 33 (21%) | 79 (19%) | |
| Janus kinase inhibitors | 3 (6%) | 29 (5%) | 24 (6%) | 8 (4%) | 13 (8%) | 19 (4%) | |
| Rituximab | 6 (12%) | 22 (4%) | 20 (5%) | 8 (4%) | 9 (6%) | 19 (4%) | |
| Abatacept | 1 (2%) | 16 (3%) | 11 (3%) | 6 (3%) | 3 (2%) | 14 (3%) | |
| Interleukin-6 inhibitors | 1 (2%) | 6 (1%) | 3 (1%) | 4 (2%) | 3 (2%) | 4 (1%) | |
| Mycophenolate mofetil | 5 (10%) | 7 (1%) | 11 (3%) | 1 (1%) | 1 (1%) | 11 (3%) | |
| Glucocorticoid use‡ | 23 (47%) of 49 | 140 (26%) of 529 | 113 (29%) of 385 | 49 (28%) of 177 | 58 (36%) of 159 | 105 (25%) of 416 | |
| Unknown | 1 (2%) | 3 (1%) | 3 (1%) | 3 (2%) | 0 | 7 (2%) | |
| Median dose (IQR), mg/day | 5 (5–8) | 5 (5–10) | 5 (5–7·5) | 5 (5–10) | 5 (5–5) | 5 (5–10) | |
| Glucocorticoid use and no DMARDs† | 3 (6%) of 49 | 22 (4%) of 529 | 19 (5%) of 385 | 6 (3%) of 177 | 13 (8%) of 159 | 12 (3%) of 416 | |
Data are n (%), unless specified. Anti-CCP=anti-cyclic citrullinated peptide antibody.
Positivity either to rheumatoid factor or anti-CCP.
The calculation excludes missing or unknown data.
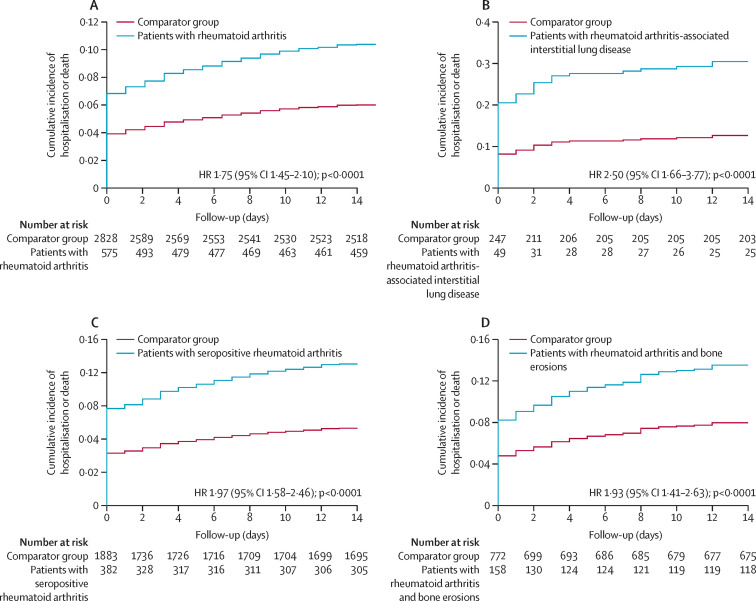
126 (22%) of 582 patients with rheumatoid arthritis (follow-up of 41 411 person-days), and 363 (13%) of 2875 comparators (follow-up of 226 550 person-days) had severe outcomes (table 3 ). The corresponding rate of severe COVID-19 was 3·04 (95% CI 2·51–3·57) per 1000 person-days among patients with rheumatoid arthritis versus 1·60 (1·44–1·77) per 1000 person-days among comparators (p<0·0001; figure 1A ). In adjusted analyses for age, sex, race, and smoking status, patients with rheumatoid arthritis had increased risk of severe COVID-19 compared with patients without rheumatoid arthritis (adjusted HR 1·75 [95% CI 1·45–2·10]; p<0·0001). These associations were slightly attenuated when adjusting for BMI and comorbidity burden (1·60 [1·31–1·95]; p<0·0001). The risk of severe COVID-19 among women with rheumatoid arthritis was two-fold higher than women without rheumatoid arthritis (2·03 [1·62–2·55]). Men with rheumatoid arthritis had no difference in their risk compared with men without rheumatoid arthritis (1·23 [0·88–1·74]). Results stratified by sex are shown in appendix (p 2). A sensitivity analysis of patients with rheumatoid arthritis and comparators who had COVID-19 before vaccine availability yielded similar results to the primary analysis (appendix p 3).
Table 3.
The association of rheumatoid arthritis with the risk of severe COVID-19 and other outcomes
| Patients with rheumatoid arthritis (n=582) | Patients without rheumatoid arthritis (comparator group; n=2875) | ||
|---|---|---|---|
| Severe COVID-19, n (%) | 126 (22%) | 363 (13%) | |
| Follow-up time, person-days of follow-up | 41 411 | 226 550 | |
| Rate (95% CI), per 1000 person-days | 3·04 (2·51 to 3·57) | 1·60 (1·44 to 1·77) | |
| Rate difference (95% CI), per 1000 person-days | 1·44 (0·88 to 2·00) | .. | |
| Unadjusted | 1·83 (1·54 to 2·17) | .. | |
| Adjusted main model | 1·75 (1·45 to 2·10) | .. | |
| Adjusted mediators model | 1·60 (1·31 to 1·95) | .. | |
| Hospitalisation, n (%) | 121 (21%) | 355 (12%) | |
| Follow-up time, person-days follow-up | 41 670 | 227 229 | |
| Rate (95% CI), per 1000 person-days | 2·90 (2·39 to 3·42) | 1·56 (1·40 to 1·73) | |
| Rate difference (95% CI), per 1000 person-days | 1·34 (0·80 to 1·88) | .. | |
| Unadjusted | 1·69 (1·43 to 2·00) | .. | |
| Adjusted main model | 1·62 (1·36 to 1·94) | .. | |
| Adjusted mediators model | 1·51 (1·25 to 1·82) | .. | |
| Deaths, n (%) | 26 (4%) | 59 (2%) | |
| Follow-up time, person-days follow-up | 50 771 | 254 623 | |
| Rate (95% CI), per 1000 person-days | 0·51 (0·32 to 0·71) | 0·23 (0·17–0·29) | |
| Rate difference (95% CI), per 1000 person-days | 0·28 (0·07 to 0·49) | .. | |
| Unadjusted | 2·31 (1·53 to 3·48) | .. | |
| Adjusted main model | 1·79 (1·14 to 2·82) | .. | |
| Adjusted mediators model | 1·53 (0·94 to 2·48) | .. | |
| Mechanical ventilation, n (%) | 17 (3%) | 55 (2%) | |
| Follow-up time, person-days follow-up | 50 946 | 254 090 | |
| Rate (95% CI), per 1000 person-days | 0·33 (0·18 to 0·49) | 0·22 (0·16 to 0·27) | |
| Rate difference (95% CI), per 1000 person-days | 0·12 (−0·05 to 0·29) | .. | |
| Unadjusted | 1·53 (0·95 to 2·41) | .. | |
| Adjusted main model | 1·49 (0·86 to 2·58) | .. | |
| Adjusted mediators model | 1·26 (0·69 to 2·30) | .. | |
Data are HR (95% CI), unless specified. Adjusted main model for age, sex, race, and smoking. Adjusted mediators model for age, sex, race, smoking, body-mass index, and Charlson comorbidity index (dichotomised as <2 or ≥2). HR=hazard ratio.
Figure 1.
Cumulative incidence curves for severe COVID-19 in patients with rheumatoid arthritis versus patients without rheumatoid arthritis (comparator group)
(A) All patients with rheumatoid arthritis. (B) Patients with rheumatoid arthritis-associated interstitial lung disease. (C) Patients with seropositive rheumatoid arthritis. (D) Patients with rheumatoid arthritis and erosive disease. HR was adjusted for age, sex, race, and smoking status. HR=hazard ratio.
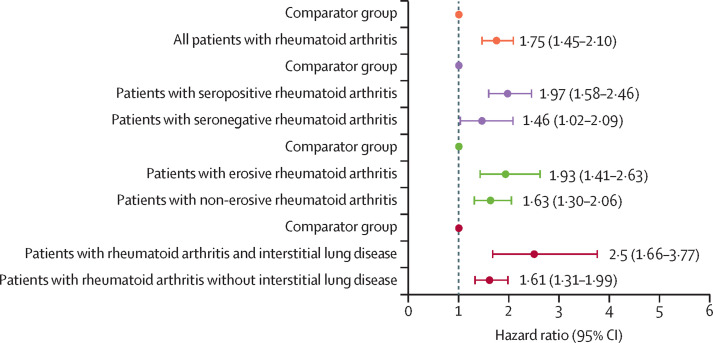
Compared with patients without rheumatoid arthritis, patients with rheumatoid arthritis-associated interstitial lung disease (adjusted HR 2·50 [95% CI 1·66–3·77]; figure 1B) or those who were seropositive (1·97 [1·58–2·46]; figure 1C) had a higher risk of severe COVID-19 (figure 2 and appendix pp 4–5). A similar association was seen among patients with erosive rheumatoid arthritis when compared with patients without rheumatoid arthritis (1·93 [1·41–2·63]; figure 1D). To further investigate these observations, we assessed the individual effect of each phenotype of rheumatoid arthritis (rheumatoid arthritis-associated interstitial lung disease, seropositive rheumatoid arthritis, or erosive rheumatoid arthritis) on severe COVID-19 by categorising patients into eight mutually exclusive groups; we found that all phenotypes with interstitial lung disease had a higher risk of severe COVID-19 outcomes than their matched comparators without rheumatoid arthritis (rate difference range 4·45–20·22 per 1000 person-days vs 0·63–2·35 per 1000 person-days). These differences were less obvious in the non-interstitial lung disease phenotypes (appendix pp 6–11).
Figure 2.
Multivariable HRs for severe COVID-19 outcomes, comparing all rheumatoid arthritis and subgroups by serostatus, bone erosions, and interstitial lung disease with matched comparators of patients without rheumatoid arthritis
Cox model adjusted for age, sex, race, and smoking status. p=0·046 for heterogeneity in the model comparing patients with rheumatoid arthritis with and without interstitial lung disease to comparators.. HR=hazard ratio.
Similarly, among patients with rheumatoid arthritis, there was a significant association of having interstitial lung disease with risk of severe COVID-19 versus having no interstitial lung disease (adjusted HR 2·61 [95% CI 1·52–4·47]) but there was no association when comparing seropositive patients (1·21 [0·71–2·05]) to seronegative patients, or when comparing patients with erosive disease (1·16 [0·81–1·66]) to patients without erosive disease (appendix p 8). Patients with rheumatoid arthritis positive for rheumatoid factor or anti-CCP had similarly increased risk of severe COVID-19 compared with patients without rheumatoid arthritis (appendix p 10).
We also assessed the association of rheumatoid arthritis with individual components of severe COVID-19 (hospitalisation or death) and with requirement for mechanical ventilation. 121 (21%) of 582 patients with rheumatoid arthritis were hospitalised and 26 (4%) died, compared with 355 (12%) 2875 of comparators who had been hospitalised and 59 (2%) comparators who had died (table 3). The corresponding rate of hospitalisations was 2·90 (95% CI 2·39–3·42) per 1000 person-days among patients with rheumatoid arthritis versus 1·56 (1·40–1·73) per 1000 person-days among the comparator group (p<0·0001). The mortality rates were 0·51 (0·32–0·71) per 1000 person-days among patients with rheumatoid arthritis versus 0·23 (0·17–0·29) per 1000 person-days among the comparator group (p<0·0001). In the models adjusted for age, sex, race, and smoking status, the risk of hospitalisation showed an increase in patients with rheumatoid arthritis versus comparators (adjusted HR 1·62 [95% CI 1·36–1·94]; p<0·0001); this association was not attenuated after adjustment for BMI and comorbidities (table 3). There were 17 (3%) patients with rheumatoid arthritis and 55 (2%) in the comparator group who required mechanical ventilation. The estimated rate for mechanical ventilation was 0·33 (95% CI 0·18–0·49) per 1000 person-days for patients with rheumatoid arthritis versus 0·22 (95% CI 0·16–0·27) per 1000 person-days for the comparator group (p=0·083). In the adjusted analyses, the risk of mechanical ventilation was similar (adjusted HR 1·49 [95% CI 0·86–2·58]; p=0·15) between patients with rheumatoid arthritis and the comparator group (table 3). In analyses restricted to patients with rheumatoid arthritis (ILD vs non-ILD, seropositive vs seronegative, erosive vs non-erosive disease), findings regarding secondary outcomes were similar to those observed in primary analyses (appendix p 8–9).
Discussion
In this large, retrospective, comparative, multicentre cohort study, we found that patients with rheumatoid arthritis had a higher risk of severe COVID-19 outcomes than had patients without rheumatoid arthritis. This association was observed across rheumatoid arthritis phenotypes but was particularly strong among patients with rheumatoid arthritis-associated interstitial lung disease. The higher risk of severe COVID-19 outcomes persisted among patients with rheumatoid arthritis-associated interstitial lung disease when compared with patients with rheumatoid arthritis without interstitial lung disease and when we examined individual COVID-19 outcomes (eg, hospitalisation and death). Our findings have important implications for the management of patients with rheumatoid arthritis during the ongoing COVID-19 pandemic. Having rheumatoid arthritis should be considered a risk factor for severe COVID-19, but the severity of this risk might vary across phenotypes.
Our findings expand on observations previously reported in studies of patients with rheumatoid arthritis and COVID-19. In contrast to OpenSAFELY in the UK,21 one of the earliest studies observing an association of rheumatoid arthritis with death due to COVID-19, we examined patients with rheumatoid arthritis specifically rather than grouping them with patients with systemic lupus erythematosus or psoriasis, and we investigated the association of specific phenotypes of rheumatoid arthritis with severe COVID-19 outcome risk. A study by Raiker and colleagues3 used TriNetX in the USA to compare outcomes among patients with rheumatoid arthritis versus general population comparators using an exposure score-matched analysis. Raiker and colleagues3 did not observe significant differences between these two populations, which was presumably driven by the inclusion of comorbidities, such as interstitial lung disease, in their exposure score. A study from Denmark22 found an increased risk of COVID-19 hospitalisation among patients with rheumatoid arthritis, regardless of vaccination status compared with matched patients without rheumatoid arthritis.
We found an increased risk of severe COVID-19 among women with rheumatoid arthritis compared with women without rheumatoid arthritis, but we did not see this among men with rheumatoid arthritis versus men without rheumatoid arthritis. This difference might be the result of factors (eg, smoking status, obesity, and comorbidities) more common in male comparators that also increased the risk of severe disease; alternatively, the proportion of men in our cohort was smaller, so our study might have lacked power to detect differences.21, 23 Indeed, a study by England and colleagues7 using the US Veterans Affairs rheumatoid arthritis cohort, which is enriched for men, also examined the risk of COVID-19 hospitalisation or death among patients with rheumatoid arthritis versus patients without rheumatoid arthritis from the Veterans Affairs system. They found that rheumatoid arthritis was associated with a 35% higher risk, especially among those patients with rheumatoid arthritis using DMARDs or glucocorticoids.7 Unlike the study by England and colleagues,7 our study examined these outcomes among a more generalisable rheumatoid arthritis population, in which the majority of patients were women rather than men. Additionally, we showed that this association was particularly strong among patients with rheumatoid arthritis and interstitial lung disease.
Among patients with rheumatoid arthritis-associated interstitial lung disease, a particularly high risk of severe COVID-19 might be the result of the parenchymal lung disease or exposure to medications (eg, rituximab or mycophenolate mofetil) often used to treat patients with rheumatoid arthritis and interstitial lung disease and known to be associated with poor COVID-19 outcomes.2, 24, 25, 26 Since patients in the comparator group were not on immunosuppressive medications, we were unable to examine the influence of baseline use of medications on COVID-19 severity. Rheumatoid arthritis disease activity might be a risk factor for rheumatoid arthritis-associated interstitial lung disease and bone erosions and for severe outcomes of COVID-19.27 Given the retrospective nature of our study, we did not have details of disease activity, so this is an avenue for future investigation. Other potential explanations for the observed associations include other comorbidities or shared risk factors (eg, smoking), but our findings mostly persisted when we accounted for these differences in adjusted models.
Previous studies have indicated that interstitial lung disease, regardless of the presence of a systemic rheumatic disease, is associated with an increased risk of COVID-19. A claims-based case-control study using the South Korean National Health Insurance database found that people with interstitial lung disease were two-fold more likely to have COVID-19 than people without interstitial lung disease and were more likely to have severe COVID-19.28 In that study, the association of interstitial lung disease due to connective tissue disease with severe COVID-19 did not persist after adjusting for comorbidities, which might be related to reduced sample size, and after adjusting for mediators that can attenuate the association, as we observed. Another study of patients with interstitial lung disease who contracted COVID-19 has described a more than three-fold increased likelihood of death compared with patients without interstitial lung disease.13 To our knowledge, our study is the first to assess the effect of rheumatoid arthritis-associated interstitial lung disease on severe COVID-19 outcomes. We investigated this effect, partly by identifying eight mutually exclusive rheumatoid arthritis phenotypes, some of which were small in sample size; our findings should be confirmed in larger cohorts. Due to small sample size, we were unable to pursue analyses based on the interstitial lung disease subtype or underlying severity. It is possible that clinicians might have had a lower threshold to hospitalise patients with rheumatoid arthritis-associated interstitial lung disease. However, we also found increased risk of the separate outcomes of mechanical ventilation and death, so we think this possibility is unlikely to explain the results.
Our study has several important strengths. First, we assembled these cohorts from more than 30 sites from two health systems in five US states. Second, because of the linkage with electronic health records, we were able to confirm rheumatoid arthritis diagnoses using validated criteria, and we were able to phenotype patients with rheumatoid arthritis according to whether or not they had interstitial lung disease, bone erosions, or autoantibodies associated with rheumatoid arthritis. Third, our study is the first to investigate rheumatoid arthritis-associated interstitial lung disease that has been verified by medical record review. The rheumatoid arthritis-associated interstitial lung disease prevalence of 9% is in line with previous studies,29 suggesting that our findings should be generalisable. Fourth, we matched each patient with rheumatoid arthritis to patients without rheumatoid arthritis as comparators by SARS-CoV-2 test date so that each case-comparator unit was in a similar phase of the pandemic regarding emergence of SARS-CoV-2 variants, testing, treatment, and vaccine availability.
Despite these strengths, our study has some limitations. First, some minority racial and ethnic groups might be under-represented, restricting the generalisability of our findings to more diverse populations. Previous studies have established that racial and minority ethnic groups have a higher risk of severe COVID-19.30, 31 Second, because of the small number of patients with rheumatoid arthritis and interstitial lung disease, we were unable to do additional analyses evaluating the impact of differences in DMARDs usage, such as B-cell depleting agents, on the observed associations. Third, there were relatively few mechanical ventilation and mortality outcomes, which might have restricted our ability to detect differences. Fourth, the majority of the infections occurred before the wide availability of vaccines against SARS-CoV-2, and a small proportion of the patients were vaccinated before their SARS-CoV-2 infection, limiting the generalisability of our findings to patients who are fully vaccinated. However, cases and comparators were tightly matched by calendar date, meaning that temporal changes in the prevention and treatment of COVID-19 were unlikely to explain results. In addition, the sensitivity analysis looking at the prevaccine period provided similar results to the main analysis. Breakthrough infection after vaccination was uncommon during our study period,32 and COVID-19 vaccine uptake has been similar between patients with autoimmune rheumatic diseases (eg, systemic lupus erythematosus) and the general population.33 Future studies are needed to assess whether risk of severe COVID-19 in patients with rheumatoid arthritis persists after vaccination. Fifth, we were unable to condition our study on the whole population of people with rheumatoid arthritis, rather than those with rheumatoid arthritis and COVID-19, because phenotyping data on rheumatoid arthritis-associated interstitial lung disease and bone erosions were done manually and thus were not available for the entire rheumatoid arthritis population. This method might have introduced collider bias34 and our results showing increased risk might be conservative. To avoid possible collider bias, a cohort study of all patients with rheumatoid arthritis previous to SARS-CoV-2 infection onset would be preferred. Since we required both patients with rheumatoid arthritis and those in the comparator group to test positive for SARS-CoV-2, there might be selection bias related to risk factors for infection and test indication (including rheumatoid arthritis phenotypes).35 However, the proportion of patients with rheumatoid arthritis and interstitial lung disease (9%), seropositivity (68%), and bone erosions (27%) were similar to previous rheumatoid arthritis studies,29, 36, 37 arguing that our results should be generalisable. Although collider bias is unlikely to fully explain our findings, future work should carefully consider the risk of introducing this bias into studies.
In conclusion, rheumatoid arthritis was associated with an increased risk of severe COVID-19 outcomes compared with the non-rheumatoid arthritis population. There was evidence that interstitial lung disease might particularly predispose patients with rheumatoid arthritis to severe COVID-19 outcomes, even more so than seropositivity or erosive disease. These findings suggest that interstitial lung disease, or its treatment, might be a major contributor to severe COVID-19 outcomes in patients with rheumatoid arthritis; however, all patients with rheumatoid arthritis should be considered to be at increased risk of severe COVID-19 outcomes.
Data sharing
Deidentified data are available after reasonable request and ethical approval to the corresponding author.
Declaration of interests
NJP reports consulting fees from FVC Health, unrelated to this work; and is supported by the US National Institutes of Health Ruth L Kirschstein Institutional National Research Service Award (T32-AR-007258). JAS has received research support from Bristol Myers Squibb; consulted for AbbVie, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Gilead, Inova Diagnostics, Janssen, Optum, and Pfizer, unrelated to this work; and is supported by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01 AR077607, P30 AR070253, and P30 AR072577) and the R Bruce and Joan M Mickey Research Scholar Fund. ZSW reports research support from Bristol-Myers Squibb and Principia/Sanofi; consulting fees from Viela Bio, Horizon, Zenas Biopharma, Shionogi, Sanofi, and MedPace, unrelated to this work; and is supported by the US National Institute of Arthritis and Musculoskeletal and Skin Diseases (K23AR073334 and R03AR078938), the Rheumatology Research Foundation, and Massachusetts General Hospital Department of Medicine. AD-G has received unrelated grant funding from the US Centers for Disease Control and Prevention, the Rheumatology Research Foundation Career Development Award, and the Robert D and Patricia E Kern Center for the Science of Health Care Delivery. All other authors have no competing interests. The content is solely the responsibility of the authors and does not necessarily represent the official views of Harvard University, its affiliated academic health-care centres, or the US National Institutes of Health.
Acknowledgments
Acknowledgments
None.
Contributors
GF-P, ELG, ZSW, AD-G, and JAS contributed to the study conception and design. Material preparation and data collection were done by GF-P, ELG, MOV-A, SV, MRN, NJP, CC, XF, RH, GCM, MAD, LM, KMMV, EK, and GQ. Analyses of data were done by XF, YZ, ZSW, and JAS. Interpretation of results was done by GF-P, ELG, NJP, YZ, ZSW, AD-G and JAS. MRN, ZSW, and JAS directly accessed and verified the underlying data. The first draft of the manuscript was written by GF-P and ELG. All authors read and approved the final manuscript.
Supplementary Material
References
- 1.Figueroa-Parra G, Aguirre-Garcia GM, Gamboa-Alonso CM, Camacho-Ortiz A, Galarza-Delgado DA. Are my patients with rheumatic diseases at higher risk of COVID-19? Ann Rheum Dis. 2020;79:839–840. doi: 10.1136/annrheumdis-2020-217322. [DOI] [PubMed] [Google Scholar]
- 2.Strangfeld A, Schäfer M, Gianfrancesco MA, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2021;80:930–942. doi: 10.1136/annrheumdis-2020-219498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Raiker R, DeYoung C, Pakhchanian H, et al. Outcomes of COVID-19 in patients with rheumatoid arthritis: a multicenter research network study in the United States. Semin Arthritis Rheum. 2021;51:1057–1066. doi: 10.1016/j.semarthrit.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Faye AS, Lee KE, Laszkowska M, et al. Risk of adverse outcomes in hospitalized patients with autoimmune disease and COVID-19: a matched cohort study from New York City. J Rheumatol. 2021;48:454–462. doi: 10.3899/jrheum.200989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pablos JL, Galindo M, Carmona L, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020;79:1544–1549. doi: 10.1136/annrheumdis-2020-218296. [DOI] [PubMed] [Google Scholar]
- 6.Kroon FPB, Najm A, Alunno A, et al. Risk and prognosis of SARS-CoV-2 infection and vaccination against SARS-CoV-2 in rheumatic and musculoskeletal diseases: a systematic literature review to inform EULAR recommendations. Ann Rheum Dis. 2022;81:422–432. doi: 10.1136/annrheumdis-2021-221575. [DOI] [PubMed] [Google Scholar]
- 7.England BR, Roul P, Yang Y, et al. Risk of COVID-19 in rheumatoid arthritis: a national veterans affairs matched cohort study in at-risk individuals. Arthritis Rheumatol. 2021;73:2179–2188. doi: 10.1002/art.41800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bower H, Frisell T, di Giuseppe D, et al. Effects of the COVID-19 pandemic on patients with inflammatory joint diseases in Sweden: from infection severity to impact on care provision. RMD Open. 2021;7 doi: 10.1136/rmdopen-2021-001987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Y, D'Silva KM, Jorge AM, et al. Increased risk of COVID-19 in patients with rheumatoid arthritis: a general population-based cohort study. Arthritis Care Res (Hoboken) 2022;74:741–747. doi: 10.1002/acr.24831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Guan WJ, Liang WH, Zhao Y, et al. Comorbidity and its impact on 1590 patients with COVID-19 in China: a nationwide analysis. Eur Respir J. 2020;55 doi: 10.1183/13993003.00547-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Zheng Y, Gou X, et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alqahtani JS, Oyelade T, Aldhahir AM, et al. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PLoS One. 2020;15 doi: 10.1371/journal.pone.0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Esposito AJ, Menon AA, Ghosh AJ, et al. Increased odds of death for patients with interstitial lung disease and COVID-19: a case-control study. Am J Respir Crit Care Med. 2020;202:1710–1713. doi: 10.1164/rccm.202006-2441LE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.D'Silva KM, Serling-Boyd N, Wallwork R, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot’. Ann Rheum Dis. 2020;79:1156–1162. doi: 10.1136/annrheumdis-2020-217888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Serling-Boyd N, D'Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among patients with rheumatic diseases 6 months into the pandemic. Ann Rheum Dis. 2021;80:660–666. doi: 10.1136/annrheumdis-2020-219279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hsu TY, D'Silva KM, Patel NJ, et al. Laboratory trends, hyperinflammation, and clinical outcomes for patients with a systemic rheumatic disease admitted to hospital for COVID-19: a retrospective, comparative cohort study. Lancet Rheumatol. 2021;3:e638–e647. doi: 10.1016/S2665-9913(21)00140-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 18.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 19.Bongartz T, Nannini C, Medina-Velasquez YF, et al. Incidence and mortality of interstitial lung disease in rheumatoid arthritis: a population-based study. Arthritis Rheum. 2010;62:1583–1591. doi: 10.1002/art.27405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patel NJ, D'Silva KM, Hsu TY, et al. Coronavirus disease 2019 outcomes among recipients of anti-cd20 monoclonal antibodies for immune-mediated diseases: a comparative cohort study. ACR Open Rheumatol. 2022;4:238–246. doi: 10.1002/acr2.11386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Williamson EJ, Walker AJ, Bhaskaran K, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–436. doi: 10.1038/s41586-020-2521-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cordtz R, Kristensen S, Westermann R, et al. COVID-19 infection and hospitalisation risk according to vaccination status and DMARD treatment in patients with rheumatoid arthritis. Rheumatology (Oxford) 2022 doi: 10.1093/rheumatology/keac241. published online April 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pijls BG, Jolani S, Atherley A, et al. Temporal trends of sex differences for COVID-19 infection, hospitalisation, severe disease, intensive care unit (ICU) admission and death: a meta-analysis of 229 studies covering over 10M patients. F1000 Res. 2022;11:5. doi: 10.12688/f1000research.74645.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avouac J, Drumez E, Hachulla E, et al. COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol. 2021;3:e419–e426. doi: 10.1016/S2665-9913(21)00059-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Felten R, Duret PM, Bauer E, et al. B-cell targeted therapy is associated with severe COVID-19 among patients with inflammatory arthritides: a 1-year multicentre study in 1116 successive patients receiving intravenous biologics. Ann Rheum Dis. 2022;81:143–145. doi: 10.1136/annrheumdis-2021-220549. [DOI] [PubMed] [Google Scholar]
- 26.Sparks JA, Wallace ZS, Seet AM, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: results from the COVID-19 Global Rheumatology Alliance physician registry. Ann Rheum Dis. 2021;80:1137–1146. doi: 10.1136/annrheumdis-2021-220418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sparks JA, He X, Huang J, et al. Rheumatoid arthritis disease activity predicting incident clinically apparent rheumatoid arthritis-associated interstitial lung disease: a prospective cohort study. Arthritis Rheumatol. 2019;71:1472–1482. doi: 10.1002/art.40904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lee H, Choi H, Yang B, et al. Interstitial lung disease increases susceptibility to and severity of COVID-19. Eur Respir J. 2021;58 doi: 10.1183/13993003.04125-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDermott GC, Doyle TJ, Sparks JA. Interstitial lung disease throughout the rheumatoid arthritis disease course. Curr Opin Rheumatol. 2021;33:284–291. doi: 10.1097/BOR.0000000000000787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acosta AM, Garg S, Pham H, et al. Racial and ethnic disparities in rates of COVID-19-associated hospitalization, intensive care unit admission, and in-hospital death in the United States from March 2020 to February 2021. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.30479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mathur R, Rentsch CT, Morton CE, et al. Ethnic differences in SARS-CoV-2 infection and COVID-19-related hospitalisation, intensive care unit admission, and death in 17 million adults in England: an observational cohort study using the OpenSAFELY platform. Lancet. 2021;397:1711–1724. doi: 10.1016/S0140-6736(21)00634-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cook C, Patel NJ, D'Silva KM, et al. Clinical characteristics and outcomes of COVID-19 breakthrough infections among vaccinated patients with systemic autoimmune rheumatic diseases. Ann Rheum Dis. 2022;81:289–291. doi: 10.1136/annrheumdis-2021-221326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chevet B, Figueroa-Parra G, Yang JX, et al. COVID-19 vaccine uptake among patients with systemic lupus erythematosus in the American Midwest: The Lupus Midwest Network (LUMEN) J Rheumatol. 2022 doi: 10.3899/jrheum.220220. jrheum.220220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Choi HK, Nguyen US, Niu J, Danaei G, Zhang Y. Selection bias in rheumatic disease research. Nat Rev Rheumatol. 2014;10:403–412. doi: 10.1038/nrrheum.2014.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffith GJ, Morris TT, Tudball MJ, et al. Collider bias undermines our understanding of COVID-19 disease risk and severity. Nat Commun. 2020;11 doi: 10.1038/s41467-020-19478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Myasoedova E, Davis J, Matteson EL, Crowson CS. Is the epidemiology of rheumatoid arthritis changing? Results from a population-based incidence study, 1985-2014. Ann Rheum Dis. 2020;79:440–444. doi: 10.1136/annrheumdis-2019-216694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sparks JA. Rheumatoid arthritis. Ann Intern Med. 2019;170:ITC1–IT16. doi: 10.7326/AITC201901010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified data are available after reasonable request and ethical approval to the corresponding author.