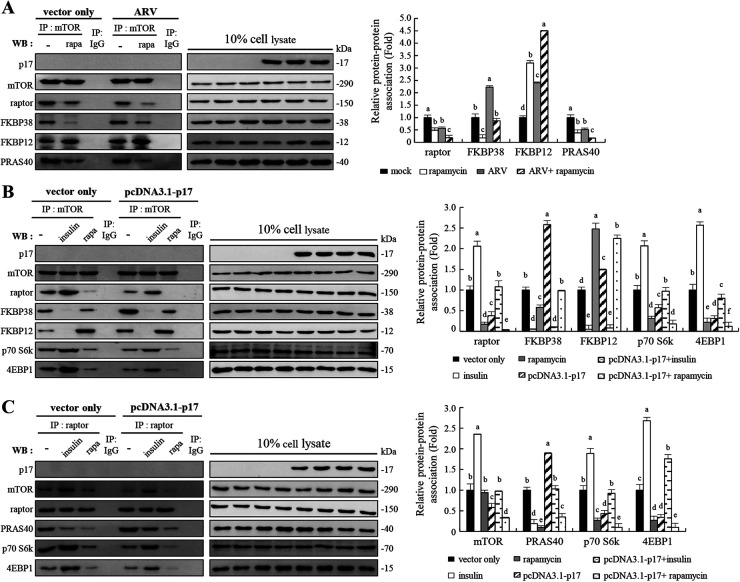
FIG 5.
The ARV p17 protein disrupts the mTORC1 assembly. (A) Coimmunoprecipitation of p17, mTOR, raptor, FKBP38, FKBP12, and PRAS40 was carried out. Vero cells were pretreated without or with rapamycin (5 μM) for 2 h, followed by infection with ARV at an MOI of 10 or without ARV infection. The cellular proteins were incubated with mTOR antibody, and the immunoprecipitated proteins were detected using the indicated antibodies by Western blotting assays. (B and C) In order to study the effects of insulin (0.2 μM) or rapamycin (5 μM) on mTORC1 assembly, coimmunoprecipitation of p17, mTOR, raptor, FKBP38, FKBP12, PRAS40, Rheb, p70 S6k, and 4EBP1 was carried out. Vero cells were pretreated with either insulin or rapamycin for 2 h, followed by transfection with either pcDNA3.1-p17 or pcDNA3.1 plasmid for 24 h, respectively. The cellular proteins were incubated with mTOR or raptor antibodies, and the immunoprecipitated proteins were detected using the indicated antibodies by Western blotting assays. Densitometry analysis results for Western blotting in panels A to C are expressed as the amount of protein and protein association (fold). Values for mock-treated cells were considered 1-fold. Signals for all blots were quantified using ImageJ software. Data in panels A, B, and C are means and SE from three independent experiments.