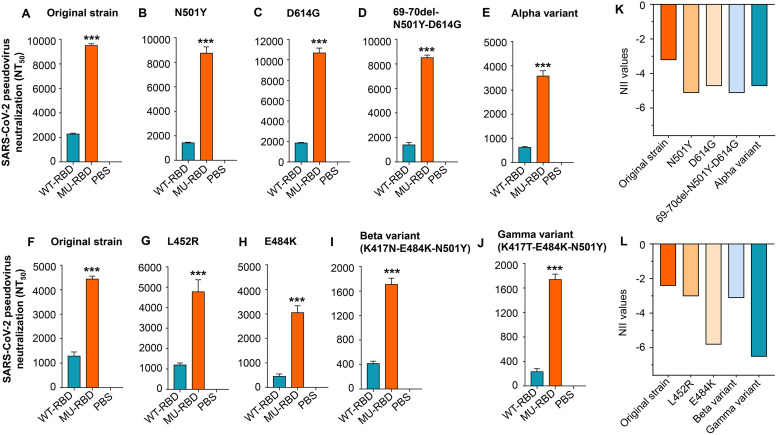
FIG 3.
Glycosylated mutant SARS-CoV-2 RBD protein elicited improved neutralizing antibodies against SARS-CoV-2 Alpha, Beta, Gamma, and Epsilon variants. hACE2-Tg mice were immunized with the prototypic WT-RBD or MU-RBD protein and boosted twice at 3 weeks. Mice injected with PBS were included as control. Mouse sera collected 10 days after the third immunization (A to E) and 10 days after the second immunization (F to J) were assessed for neutralizing activity against infection of pseudoviruses expressing S protein of the SARS-CoV-2 original strain and each variant harboring mutation(s) at the indicated amino acid(s), respectively. Alpha variant (B.1.1.7 lineage) contains all 10 amino acid mutations (69 to 70 deletion, 145 deletion, N501Y, A570D, D614G, P681H, T716I, S982A, and D1118H) in the S protein of SARS-CoV-2. Neutralizing activity was expressed as 50% neutralizing antibody titers (NT50) against pseudovirus infection in 293T cells expressing hACE2 receptor (hACE2/293T). Data are presented as mean ± SEM of quadruple wells from pooled sera of five mice in each group. ***, (P < 0.001) indicates significant differences between the MU-RBD and WT-RBD groups. The experiments were repeated twice, resulting in similar results. (K to L) Calculated neutralizing immunogenicity index (NII) values based on the neutralizing antibody titers (NT50) and the following formula: (NT50-WT – NT50-MU)/NT50-WT, where NT50-WT and NT50-MU represent NT50 induced by the WT-RBD and MU-RBD, respectively.