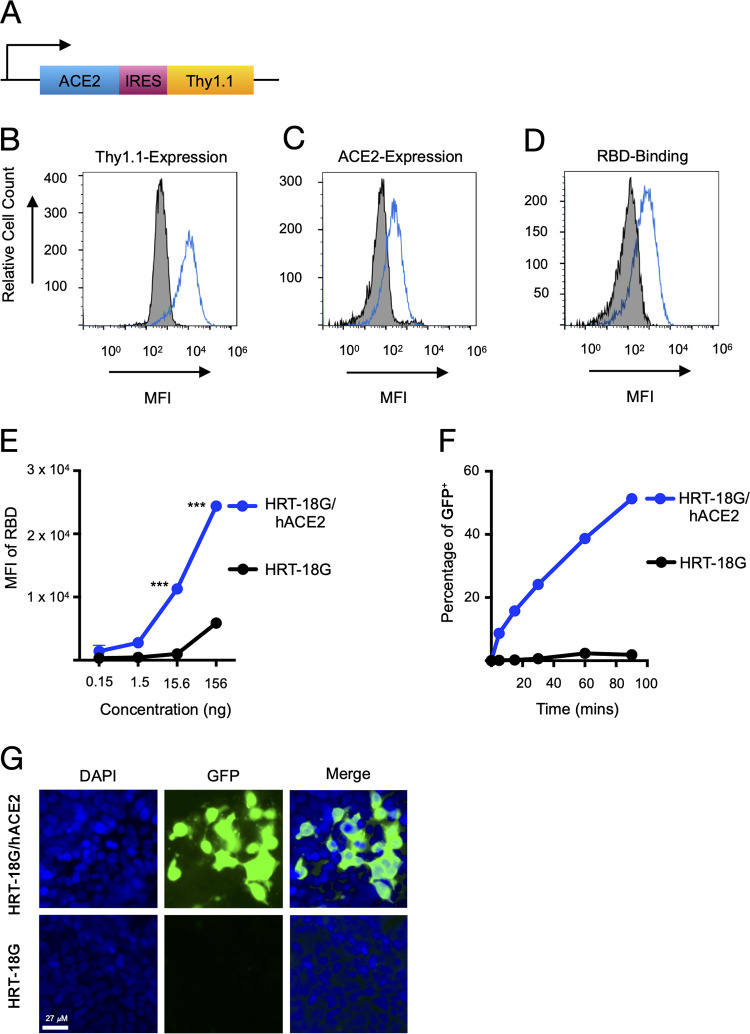
FIG 1.
Recombinant SARS-CoV-2 RBD protein binds to human ACE2 and facilitates pseudoviral infectivity. (A) Schematic of the bicistronic mRNA (3,537 bp) for the production of human ACE2 (2,415 bp; 805 amino acids [aa]) and the reporter cell surface protein mouse Thy1.1 (489 bp; 163 aa). (B) HRT-18G cells were stably transfected with a plasmid containing cDNA of hACE2 and magnetically sorted for Thy1.1 expression. HRT-18G/hACE2 (blue trace) and parental HRT-18G (shaded histogram) cells were stained with fluorescent anti-Thy1.1 antibody, and expression was analyzed by flow cytometry. (C) HRT-18G cells stably expressing ACE2 (blue trace) and parent cells (shaded histogram) were incubated with fluorescent anti-ACE2 antibody and analyzed by flow cytometry. (D) HRT-18G parental cells (shaded histogram) and HRT-18G/hACE2 cells (blue trace) were incubated with the Alexa Fluor 647-labeled SARS-CoV-2 S-protein RBD and analyzed for protein binding by flow cytometry. (E) Same as panel D except that cells were incubated with a range of concentrations of the Alexa Fluor 647-labeled SARS-CoV-2 S-protein RBD and analyzed by flow cytometry. (F) HRT-18G/hACE2 and HRT-18G cells were infected with GFP-expressing SARS-CoV-2 pseudovirus at an MOI of 10 for the indicated times, washed to remove excess virus, and incubated at 37°C overnight. Infectivity was analyzed by flow cytometry 16 h later, and the percentage of GFP-positive (GFP+) cells is reported. (G) HRT-18G/hACE2 and parent cells were infected with a GFP-expressing SARS-CoV-2 pseudovirus at an MOI of 0.5 and cultured for 36 h prior to analysis by fluorescence microscopy. Cells were stained with DAPI to delineate the nucleus. All data presented are representative of results from three independent experiments. Statistical significance is indicated (***, P < 0.0001). MFI, mean fluorescence intensity.