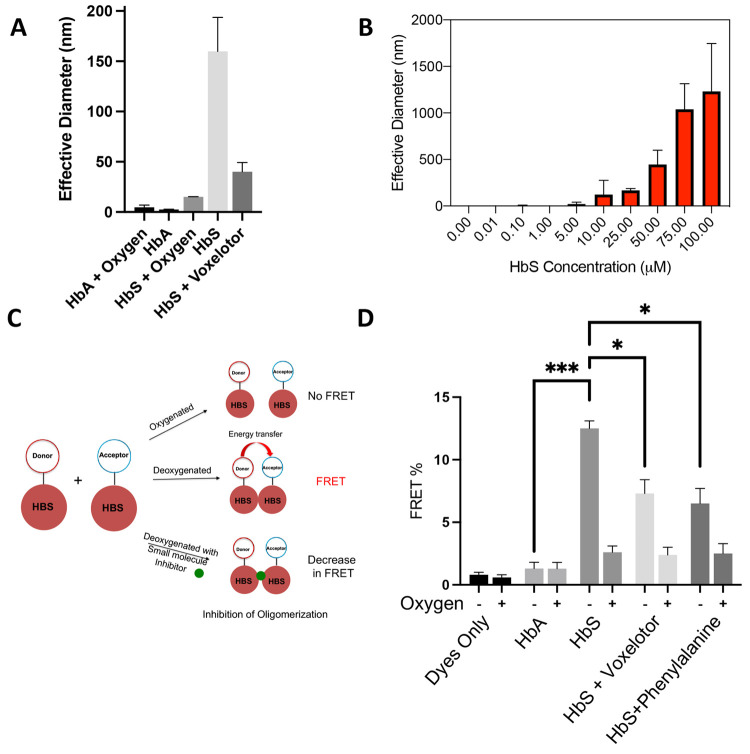
Figure 1.
Direct measurement of prefibrillar HbS oligomers by DLS and FRET. (A) Confirmation of oligomerization via DLS. Purified HbA or HbS (25 μM) was vacuum degassed with argon for 5 min, and then DLS measurements were taken at room temperature. (B) HbS oligomer size versus concentration. Purified HbS (0.01–100 μM) was degassed with argon for 5 min, and then DLS measurements were taken at room temperature. (C) Schematic of TR-FRET scheme. Time-resolved FRET can be used to probe association of deoxy-HbS monomers in solution and to discover small molecules that disrupt these assemblies. (D) FRET efficiency of fluorescent dyes, fluorescently labeled HbA and HbS samples under deoxygenated and oxygenated conditions. FRET efficiency of HbS sample was increased under deoxygenated conditions, demonstrating that the signal is specific to association of HbS in the absence of fibers. Voxelotor and phenylalanine, which are known to inhibit polymerization, reduced FRET efficiency of HbS under deoxygenated conditions. All DLS data are reported as the effective diameter of the mean assembly size. Hypothesis testing was performed using parametric ANOVA with Dunnett’s multiple comparison testing. *p-value < 0.05; **p-value < 0.01; ***p-value < 0.001.