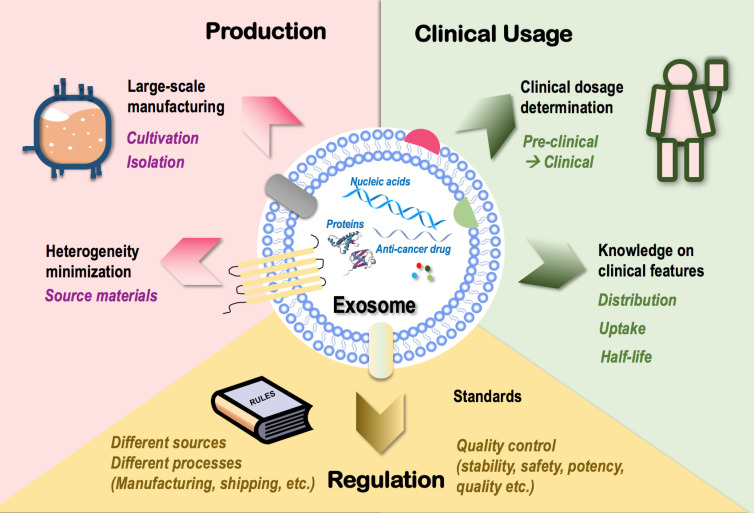
Figure 3.
Challenges limiting the clinical translation of exosomes as onco-therapeutics. Challenges limiting the clinical translation of exosomes fall into three categories, i.e., exosome production, clinical usage, and regulation. In “production,” techniques that enable large-scale exosome manufacturing such as source cell cultivation and exosome isolation, and techniques minimizing exosome heterogeneity such as control over the heterogeneity of source materials are limiting factors. In “clinical usage,” knowledge on exosome dosage for clinical use and clinical features of exosomes such as distribution, cell uptake, and half-life are limiting factors. In “regulation,” lack of a set of industrial standards feasible for the manufacturing and shipping processes of exosomes derived from different sources and lack of quality control guidelines over exosome stability, safety, potency, and quality are limiting factors.