Figure 1.
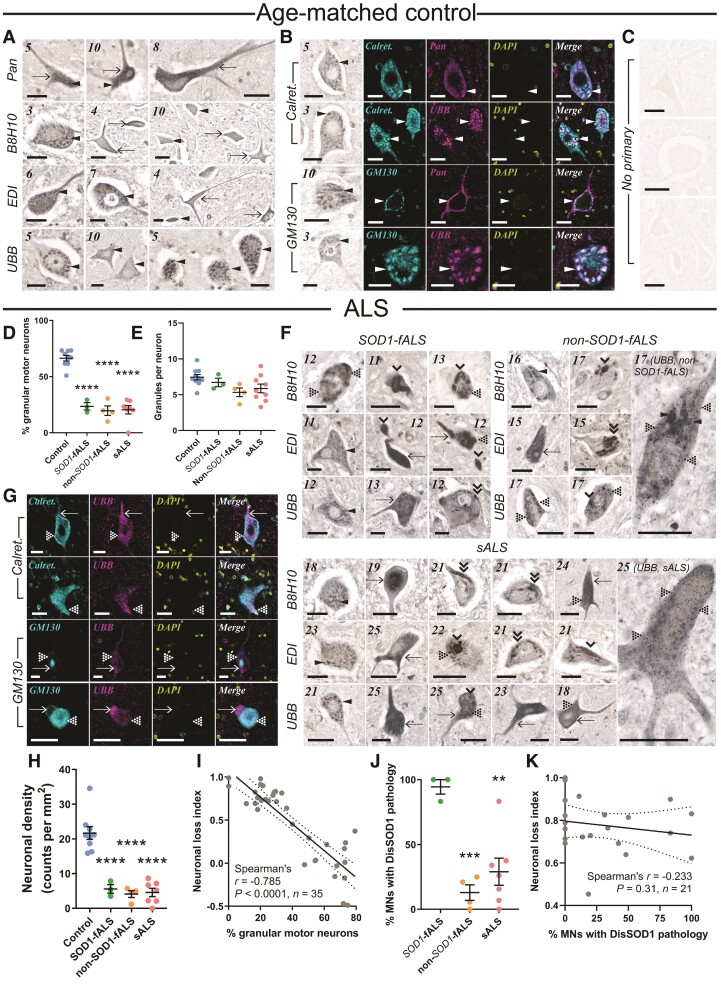
DisSOD1 accumulation, aggregation and mislocalization in the vulnerable ventral spinal cord of ALS cases. (A) Pan-SOD1 antibodies, as well as conformation-specific antibodies raised against disordered mutant SOD1 (B8H10), disSOD1 with an exposed dimer interface and disSOD1 with an unfolded β-barrel (UBB) were used to profile the distribution of mature SOD1 and disSOD1 conformers within control (n = 10) ventral spinal cord tissues. These SOD1 antibodies (Supplementary Table 3) detected granular cytoplasmic staining (arrowheads) and diffuse cytosolic staining (arrows) within control motor neurons. (B) Visualization of granular SOD1 immunostaining within control spinal cord motor neurons using the UBB conformation-specific SOD1 antibody and DAB chromogen. Granular SOD1 immunostaining strongly colocalized with the ER–Golgi markers Calreticulin and GM130 in control motor neurons. DAPI was also used to visualize cell nuclei. (C) No immunostaining was observed in spinal cord tissue sections processed as before in the absence of primary antibodies. (D) Motor neurons possessing granular SOD1 immunostaining were less abundant in all ALS subgroups compared with controls (one-way ANOVA: P < 0.0001, F = 55.82; Dunnett’s multiple comparisons post hoc tests: P < 0.0001 for all comparisons). (E) Granule numbers within surviving ALS motor neurons exhibiting granular SOD1 immunostaining were equivalent to those within control motor neurons (Kruskal–Wallis H-test, P = 0.073, H = 6.975). (F) In addition to granular and diffuse cytosolic immunostaining, conformation-specific antibodies labelled a variety of disSOD1 inclusions in ventral spinal cord motor neurons of SOD1-fALS (n = 3), non-SOD1-fALS (n = 4) and sALS (n = 9) cases; punctate inclusions (dotted arrowheads), globular inclusions (single downwards arrowheads) and fibrillar skein-like inclusions (double downwards arrowheads). Case numbers (Supplementary Table 1) are listed in the top left corner of each panel in A and F. (G) DisSOD1 conformers immunolabelled by the UBB SOD1 antibody were not colocalized with the ER–Golgi markers Calreticulin and GM130 in motor neurons of ALS cases. DAPI was also used to visualize cell nuclei. Scale bars = 25 µm in A–C, F and G. (H) Significant reductions in motor neuron density were observed in all ALS subgroups compared with controls (one-way ANOVA: P < 0.0001, F = 30.65; Dunnett’s multiple comparisons post hoc tests: P < 0.0001 for all comparisons). (I) The proportion of granular spinal cord motor neurons was correlated with great indices of motor neuronal loss in the ventral spinal cord. (J) The proportion of motor neurons (MNs) with punctate, globular and skein-like disSOD1 inclusions was higher in SOD1-fALS cases compared with non-SOD1-fALS and sALS cases (one-way ANOVA: P = 0.0011, F = 13.39; Dunnett’s multiple comparisons post hoc tests: non-SOD1-fALS: P = 0.0008; sALS: P = 0.002). Data in D, E, H and J represent mean ± SEM. *P < 0.05, ***P < 0.001, ****P < 0.0001. (K) The proportion of spinal cord motor neurons with disSOD1 inclusion pathology was not correlated with neuronal loss in the ventral spinal cord. Spearman’s r coefficient, the P-value and the number of XY pairs analysed (n) are stated within I and K. A correlation is strong if Spearman’s r = 0.5 or higher.