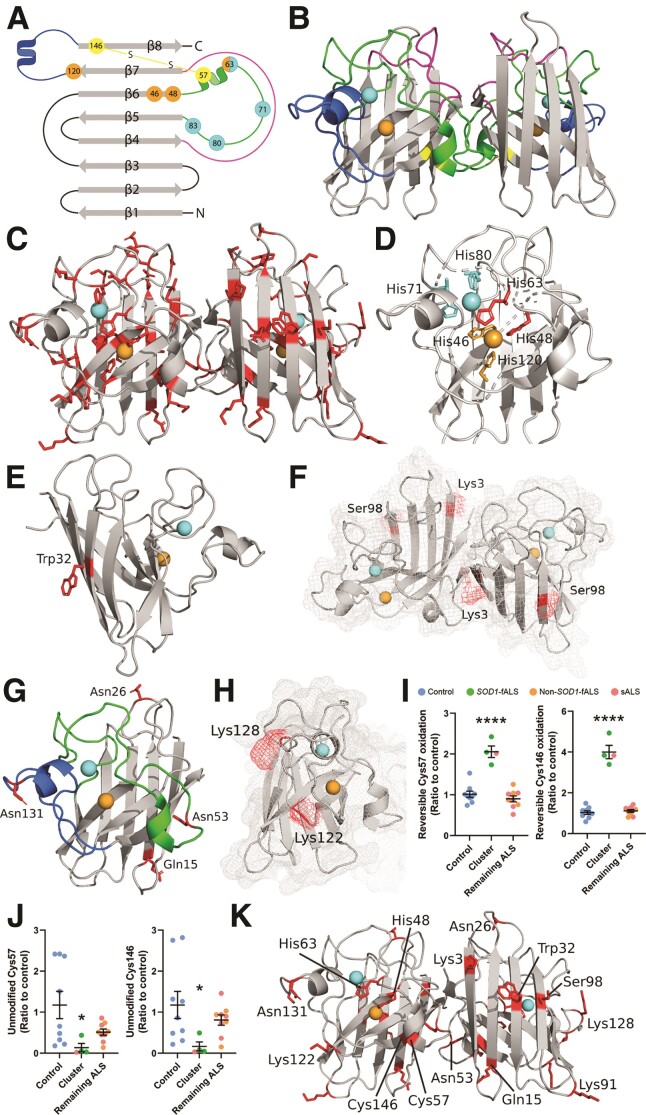
Figure 5.
SOD1 protein structure and locations of PTMs which are significantly altered in the ventral spinal cord of fALS and sALS cases. (A and B) Mature SOD1 is dimeric, with each monomer comprising an eight-stranded β-barrel (grey) with one bound Cu (orange) and Zn (cyan) ion. The electrostatic loop (blue) contains charged and polar residues important for guiding anionic superoxide towards Cu in the active site. Three histidine residues and one aspartic acid residue (cyan) within the metal-binding loop (green) facilitate Zn coordination, while four histidine residues (orange) mediate Cu coordination. The disulphide loop (yellow) is a substructure within the metal-binding loop, containing one of two cysteine residues that form an intramolecular disulphide bond within SOD1 protein (yellow). The Greek key loop (pink) forms a plug at one pole of the β-barrel and contributes to dimer interface stability. (C) Distribution of all residues identified as sites of PTMs in SOD1 protein isolated from the ventral spinal cord (VSpC) of ALS cases and controls. Significant differences in the oxidation of His48 and His63 (D) oxidation and nitration of Trp32 (E) acetylation of Lys3 (F) phosphorylation of Ser98 (F) deamidation of Gln15, Asn26, Asn53 and Asn131 (G) and in the levels of carboxyethyllysine at Lys122 and Lys128 (H) were identified between a proportion of ALS cases and controls. Residues are labelled in wild-type SOD1 using three letter amino acid codes and the side chains (D–H) of altered residues are highlighted in red. Details of specific ALS cases exhibiting alterations to each PTM are presented in Supplementary Table 9. Reversible PTMs to Cys57 and Cys146 (I) were significantly increased in SOD1 protein isolated from three SOD1-fALS cases and one sALS case (cluster), compared with controls and remaining ALS cases (one-way ANOVA: Cys 57, P < 0.0001, F = 36.72; Cys146, P < 0.0001, F = 120.2; Dunnett’s multiple comparisons post hoc tests: P < 0.0001 when comparing both Cys57 and Cys146 to controls and remaining ALS cases). Corresponding significant reductions in unmodified Cys57 and Cys146 (J) residues were also identified in these cases compared with controls and remaining ALS cases (Kruskal–Wallis H-test with Dunn’s multiple comparisons post hoc tests for both residues; Cys 57—cluster versus control: P = 0.03; Cys146—cluster versus control: P = 0.04). Complete details of statistical analyses presented in I and J are presented in Supplementary Table 8. Data in I and J represent mean ± SEM. *P < 0.05, ****P < 0.0001. (K) Distribution of all residues whose post-translational modification is significantly altered in ALS cases.