Abstract
Background
Two remote First Nations communities each collaborated with an urban-based liver clinic to organize wide-spread testing, followed by linkage to care for hepatitis C virus (HCV).
Method
Involvement of community members was central to planning and conduct of the programs. Samples were obtained using dry blood spot cards (DBS). A week-long pilot study in Community 1 investigated the effectiveness of the program, using DBS. Community 2, being larger, more remote, and known to be endemic for HCV was more challenging. Three-week-long testing drives plus a stand-alone testing day were used to collect samples over 5 months. Public Health Agency (PHAC)’s National Laboratory for HIV Reference Services (NLHRS) received and tested the DBS samples for HCV and other blood-borne infections. Outcomes were measured by number of people tested, the quality of the tests, and community members’ satisfaction with the program and retained knowledge about HCV, based on interviews.
Results
In Community 1, 226 people were tested for HCV over 4 days. 85% agreed to human immunodeficiency virus (HIV) testing as well. In Community 2, 484 people, one-half of the adult population, were tested. Surveys of participants showed food was the most significant draw, and Facebook the most effective way to inform people of the events. Interviews with staff and participants showed a high level of satisfaction.
Conclusion
The results suggest this is an effective approach to testing for HCV in unusually challenging settings. Lessons from the program include the power of community involvement; and the effectiveness of a highly targeted health initiative when developed through collaboration.
Keywords: community-led initiatives, dried blood spot (DBS), First Nations health, VIRCAN
Introduction
Before the arrival of COVID-19, hepatitis C virus (HCV) was the deadliest infection in Ontario, as measured by years of life lost (1). While vaccination is addressing the spread of COVID-19, there is no vaccine for HCV, with no likelihood of one being developed in the near future, due to the remarkable variability of the virus.
In terms of detection, HCV produces no symptoms for the majority of those exposed until the later stages of the infection, allowing the disease to remain hidden for years while it slowly destroys the liver and potentially spreads to others. A recent study in Ontario found almost a third of those diagnosed with HCV are not discovered until they have life-threatening complications (2). HCV is spread blood-to-blood, such as by sharing needles, or formerly, through blood transfusion, being 10x more infectious by blood than human immunodeficiency virus (HIV) (3).
The World Health Organization (WHO) has set a goal of eliminating infectious hepatitis B and C as epidemic diseases by 2030 (4); similarly, the 2017 World Indigenous People’s Conference on Viral Hepatitis issued a resolution to eliminate viral hepatitis from Indigenous Peoples and Tribal Communities worldwide by 2030 (5). The prevalence of HCV has been reported to be higher among Indigenous Peoples in Canada with a study including regional health authorities across the country from 1999–2004 reporting the incidence of HCV to be 6.8x higher in Indigenous versus non-Indigenous Canadians (6).
Since that study, the situation has likely worsened in Ontario for Indigenous communities. An alarming expansion of opiate use in Northwest Ontario prompted local chiefs to declare a state of emergency in 2009 (7). Estimates of non-prescription opiate use in Ontario’s Northwest First Nations (FNs) are as high as 41% to 75% in the adult populations (8,9), suggesting HCV infection is likely to be a serious and growing problem in the region. Preliminary testing of these communities confirms a very rapid rise in HCV positive cases in recent years (10).
While there are no vaccines for HCV, highly effective treatments have been developed in the last decade. Because of these advances, the major obstacle to arresting HCV spread is detection and linkage to care. A conventional approach in isolated communities might be to post invitations to be tested, with limited results; or to introduce a specialized testing team from outside, which would be expensive and possibly ineffective.
A third alternative takes advantage of the isolated, relatively close-knit identity of each community by recruiting community members to organize, tailor, and carry out testing. Here we present an approach based on close collaboration between the Toronto Centre for Liver Disease (TCLD)’s Viral Hepatitis Care Network (VIRCAN) and the local governance and community members of two First Nations in northwest Ontario. We hypothesize that this approach, coupled with the use of dried blood spots (DBS) as a simple sample collection method, is effective for HCV testing, and for increasing community awareness about HCV. This community-wide, health-based approach to HCV, rather than a targeted risk-factor approach, is in the spirit of the principles of Two-Eyed Seeing outlined in earlier work on HCV treatment (11).
Community differences
There were important differences between the two communities.
Community 1 was considered to be the less-challenging place to execute the pilot program. While still remote, it is within a 40-minute drive of a large town with all amenities: a hospital, supermarket, department store, printing shop, and computer store. The goal of the program in the first community was a proof of principle that DBS could be effectively employed at a community-wide level, and that locally-developed advertising with incentives could effectively motivate the population to participate in the program.
Because of the close proximity of a hospital, the local health director reassigned nurses of the local clinics for three days to focus on the testing drive. Nurses remained available and on-call should the need arise, and the local doctor in the community remained available, as she was not involved in the testing. The director later reported there had been no negative outcomes from this decision.
Community 2, on the other hand, was fly-in only, with a larger population, hundreds of miles from the nearest town, and was expected to have a much higher burden of infection, suggested by preliminary results from earlier testing of participants in the local buprenorphine-naloxone program.
Method
The overall approach used in the two communities was similar, and can be summarized as the following: VIRCAN, part of the TCLD, was invited by the chief and council of each community to help organize community-wide testing. A member of the community was assigned or volunteered to collaborate with VIRCAN. That person formed a team of community members and local health care staff. The team discussed the approach to use: timing, duration, incentives, advertising, and special events. Advertising included: radio, Facebook, interviews, flyers, and posters. Members of both testing teams were trained in DBS collection techniques by the National Laboratory for HIV Reference Services (NLHRS) immediately before the programs began. Program participants were provided with pre-test counselling, and their personal information was printed and labelled onto each DBS card before blood was collected; samples were dried, readied for shipping, and sent to the NLHRS in Winnipeg. DBS was chosen to obtain blood from participants, as the technique could easily be taught to non-medical staff and allowed for large numbers of samples to be stored and shipped. Feedback was obtained about the initiative through interviews with community members.
Community-specific methods
Community 1
In 2016, a representative of VIRCAN presented to a Chiefs’ Council on Health on the dangers of HCV and the opportunities for cure, leading to an invitation by the chief of Lac Seul First Nation to pilot DBS sample-collection in his community for wide-spread HCV testing. After a presentation to Lac Seul Council by VIRCAN, the chief appointed the community’s health director to work on the project. The health director, his staff, a hired elder, a VIRCAN staff member, as well as members of the Sioux Lookout First Nations Health Authority (SLFNHA) developed a protocol with Ownership, Control, Access, and Possession (OCAP) and TRI-Council Policy Statement (TCPS2) principles in mind. The protocol was to increase testing for blood-borne infections and improve estimates of HCV prevalence and incidence in multiple First Nations communities in the region and included pre-and post-test counselling scripts. The protocol was approved by the Meno Ya Win Health Centre’s Research Ethics Board of Sioux Lookout before testing began. The National Laboratory for HIV Reference Services provided training in Winnipeg to Lac Seul’s health director, his staff and VIRCAN staff member, teaching the group proper DBS protocol to obtain samples for testing. To encourage people to get tested, incentives included gift cards, funded by Council, as well as food, discussed as an important element to draw people to the testing sites. The one-week event was advertising by local radio; Facebook; an announcement at the community’s Annual General Meeting; the creation of HCV awareness displays, posted in the community clinics; a posting on the band website; and the creation of an event flyer distributed to every household in the community. Testing began in January 2017.
The flyer (see Figure 1) included dates and times for the testing, an endorsement by the chief, a list of prizes, and promises of food; it went through a dozen revisions with feedback from the chief and members of the committee. Flyer delivery, along with an HCV information pamphlet from the Canadian Aboriginal AIDS Network, was organized by the local public school principal, who employed a group of her students, paid with iTunes cards.
Figure 1:

Flyer designed and distributed in Community 1
At the end of the week’s event, staff involved were asked the following questions about the experience: Are you satisfied with the preparation process; do you feel staff had meaningful input into deciding how this was conducted; do you feel things went smoothly; do you feel staff were adequately trained for the task; did you feel the people who came were a representative sample of the community?
Community 2
Community 2, Kitchenuhmaykoosib Inninuwug (KI), runs a large buprenorphine-naloxone program. The program includes HCV testing upon induction; these tests had revealed in recent years a very high prevalence of HCV exposure. The health director approached VIRCAN in 2018 to discuss community testing. Talks with chief and council, the health director and VIRCAN led to the development of a testing strategy for the community.
Organization of the program in KI was complicated by its larger size, being only accessible by air transport, the more limited medical workforce available to dedicate themselves to program, and the greater ambition of the project: to try to test the entire population. As the program in KI aimed at wide-spread testing, several weeks were planned for the initiative over the course of 2019. Due to the scope of the initiative, industry was approached to assist with funding. A grant submitted by the community was secured from Gilead (manufacturer of several current HCV medications) who provided CAD $56,900 from their Conquering Hepatitis via Micro-Elimination (CHIME) program. Gilead had no influence over the program approach used, no involvement in the program itself, and no requirement over which therapies were used for HCV treatment in the community.
Funds were used to hire a local nursing student who was instrumental in planning and carrying out the program, to train three paid community members to perform DBS sample-gathering, to purchase food and to hire local staff to cook for community gatherings, to provide small prizes for students who submitted HCV-based designs used in advertising posters (see Figure 2), and to provide small prizes raffled off to participants each testing week. Chief and council also chose to offer two grand prizes, not covered by this grant, of a hunting trip and a shopping trip.
Figure 2:

Poster with local student art distributed in Community 2
To measure the impact of the testing initiative on the community, a standardized quantitative evaluation was performed prior to community-wide testing, with the intention of repeating the evaluation sometime after the event. The evaluation, known as the Community Readiness Assessment, is a series of interviews with standardized questions conducted with multiple community members, to determine the awareness of, attitudes towards, and preparedness to deal with a public health issue in the community, in this case HCV. The results are analyzed to produce a numerical measure of readiness. The HCV Community Readiness Assessment was adapted by the Canadian Aboriginal AIDS Network (CAAN) from the Colorado State University TriEthnic Centre’s Community Readiness Assessment Model (12).
Local responsibility for organization fell to the HCV nurse and the nursing student recruited from the community. The two formed the core of the planning staff, who were later joined by a community public health nurse and assisted by a VIRCAN staff member. In Community 1, there were eight people on-site to plan and execute tasks, while in Community 2, the burden of planning and execution fell heavily on the first-two mentioned individuals. Training for DBS labelling and shipping was provided by the NLHRS in Sioux Lookout in April 2019 where it was decided samples should be labelled with Ontario Health Insurance Plan (OHIP) numbers and routed through Public Health Ontario labs before reaching the NLHRS in Winnipeg where they would be tested.
Creating awareness about the testing events was largely developed by the two-person planning team and the VIRCAN staff member. These included an educational luncheon for community elders about HCV, organized by the nursing student; a short video delivered by the chief and posted on Facebook; Facebook announcements about upcoming events; and presentations at the local school to children and staff during each week of testing. The events themselves included: week one (May) free lunches and dinners in the community hall; week two (June) free lunches and dinners, plus a walk-a-thon to raise awareness about HCV, with local radio following the progress of the walkers and encouraging people to get tested throughout the day; in August, a one-day testing event, billed as a ‘family day,’ with children’s prizes and a dunking tank; and week 3 (September), a mobile testing team visiting specific regions by van each day of the week. The team included two people to take the DBS samples, and a Band Council member to knock on doors and explain what was being offered. Other advertising included posters designed by local students, rewarded with iTunes cards. For week three, a flyer about testing, including a map of where the van would be each day, was given to students on the Monday to bring home and show their families.
During weeks one and two of the testing drives participants were offered a survey to fill out regarding the event itself, health testing in general, and education in general (see Table 3).
Table 3:
Final survey used for weeks 1 and 2 of testing
| Survey questions | No. of respondents |
|---|---|
| 1. What brought you in today? | |
| a. Food | 21 |
| b. Prizes | 7 |
| c. Radio | 7 |
| d. Facebook | 10 |
| e. Other: | 8 |
| 2. Do you know anyone who doesn’t want to be tested? | |
| Yes | 6 |
| No | 70 |
| 3. Do you feel community testing is something KI needs? | |
| Yes | 75 |
| No | 2 |
| 4. Would you like to see more community health events? | |
| Yes | 72 |
| No | 0 |
| Don’t care | 4 |
| If yes, which topics (indicate below): | |
| Blood pressure clinic | 25 |
| Healthy-Cooking classes | 40 |
| Diabetes Screening | 41 |
| New-Baby care | 23 |
| Support groups for: | 3 |
| Sexual trauma | 28 |
| Bullying | 40 |
| Grief/Loss | 44 |
| Alcohol (AA) | 29 |
| Women's support | 18 |
| Other: | |
| 5. Do we need more health/safety talks (eg in school, on radio) in KI? | |
| Yes | 69 |
| No | 4 |
| If yes, which areas? (please indicate below) | |
| Diabetes | 50 |
| Heart disease | 20 |
| Fatty liver | 19 |
| Blood pressure | 26 |
| Stroke | 20 |
| Sexual health | 32 |
| Pregnancy | 23 |
| Healthy diet | 31 |
| Family planning | 23 |
| Parenting | 33 |
| Safe injection practices | 24 |
| Mental health | 42 |
| Coping with addiction | 38 |
| Basic first aid | 28 |
| Fire safety | 34 |
Results
Community 1
In Community 1, Lac Seul, 226 people, 33% of the adult population, came to be tested for HCV; as well, 85% of those elected to get tested for HIV; and 90% for HBV. When asked, 45% chose not to be informed if all results were negative, reducing the burden on nursing staff.
Figure 3 provides a breakdown of the age and sex demographics of participants in Community 1. The initiative attracted a wide age range, the most prevalent being 26- to 30-year-olds.
Figure 3:
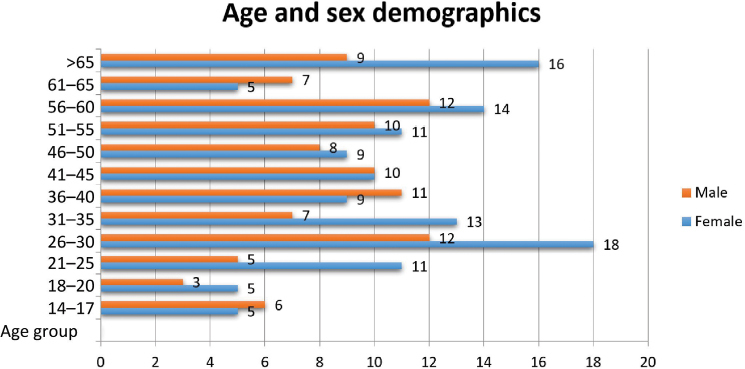
Demographics of participants in Community 1
Figure 4 shows the rate at which people were tested, illustrating a steady stream of people arriving on each day, at a rate of approximately 7–12 people per hour throughout.
Figure 4:
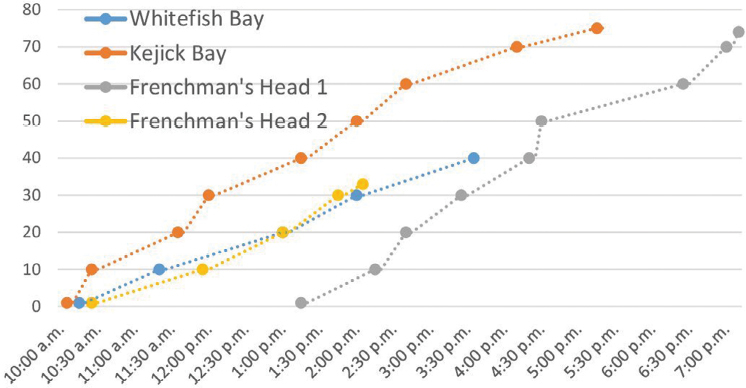
Rate of testing in Community 1
DBS samples were dried, ordered, and packed each night and delivered as a single package to the NLHRS four days after the last event; therefore, seven days passed from the time the first sample was obtained to when all samples reached the lab.
In response to the questions about their experience, staff said the work was ‘fun,’ that it was ‘a team-building’ experience, and that they felt ‘really well-trained.’ Also, ‘there was a lot of opportunity to give feedback.’ The director of the buprenorphine-naloxone program answered, ‘I very much felt the … staff had meaningful impact on how this was conducted’ (Personal correspondence).
The NLHRS said that the quality of the DBS cards they received were ‘exceptional,’ compared to other studies they’ve done with DBS (Personal correspondence).
Fewer than five people tested positive for HCV (subsequently treated); no one was positive for HBV or HIV.
Community 2
The objective with Community 2, Kitchenuhmaykoosib Inninuwug, was to test as much of the adult population as possible, and to identify and bring to care people unknowingly living with HCV. Previously, as of February 5, 2019, the local HCV nurse (one of the authors) had tested approximately 300 community members and was following 181 people positive for HCV Ab. These 300 tested included participants in the buprenorphine-naloxone program, all pregnant women coming to the nursing station, and anyone appearing at the nursing station with clinical evidence or history of injection drug use. As the existing community buprenorphine-naloxone program induction was the main avenue by which people were tested, it was unknown whether the high positivity rate found in the community was unique to the program participants or represented a high prevalence in the population in general.
A Community Readiness Assessment was made just before the community-wide testing program by the late Geri Bailey of CAAN. Her team interviewed 18 community members. The cumulative results determined the community to be at a readiness level of 3, which is described as ‘vague awareness.’
A second Community Readiness Assessment was conducted in September–October of 2020, approximately one year after the testing initiative ended. Based on 12 interviews, scored by three evaluators, the readiness had risen to 4.4. Rounded down to level 4, this readiness level is characterized as ‘Clear recognition something must be done,’ by the Assessment model. In addition, during the second assessment, after the standard questions had been answered, interviewers added the question, ‘Do you think the program affected people’s awareness of hep C as a concern?’ to which the majority responded ‘Yes.’
The outreach program gathered samples from 484 people, including 50.7% (431/849) of the adult population. Testing was done 4 days for each week of testing. With the additional ‘Family Day,’ there were a total of 13 full days of testing. The average number of people tested was 36 per day over approximately eight hours, resulting in an average influx of 4.5 people/hour. The maximum number of people tested was 54 on the day of the walk-a-thon; the minimum number was 14, coinciding with a funeral, which likely affected the turn-out.
Table 1 shows that involvement by the community remained fairly consistent from week to week, averaging 150 per week. There was a concern that only older adults would get tested, however, many younger adults were reached (Table 2). Notably, these young adults were largely reached through the door-to-door van outreach program of the final test drive.
Table 1:
Weekly breakdown of testing
| No. of participants | |
|---|---|
| Week 1 May 2019 | 159 |
| Week 2 Jun 2019 | 155 |
| Week 3 Sep 2019 | 135 |
| March training 2019 | 6 |
| Family day Aug 2019 | 29 |
Table 2:
Testing of 16- to 30-year-olds
| No. of participants | |
|---|---|
| Drive 1 | 34 |
| Drive 2 | 39 |
| Drive 3 (mobile test van) | 56 |
Survey results
The participants were surveyed about the testing program and about health education in general (see Table 3). Of the 77 respondents, 53 answered the question, ‘What brought you in today?’ Food was shown to be the primary draw, (21/53), followed by Facebook (10/53). A large majority, 75/77, agreed on the need for testing in the community; a similar number, 72/76, said they would like to see more community health events, with none objecting to such events. Most, 69/73, agreed there is an increased need for health and safety talks on the radio and in schools. There is, of course, a selection bias to these answers since the people who show up for an event have already endorsed it by their presence. With that in mind, people were also asked whether they knew of anyone who did not want to be tested, and only 6 of 76 respondents (8.0%) said yes.
484 people were tested. A review of the 2019 band list found 849 people born before 2005 living on reserve. Comparing this number to the number of people tested during the events also born before 2005 (431), we can say that more than half (431/849 or 50.7%) of the community’s adults were tested in this program. Of the 484 DBS samples obtained through the initiative, 470 were tested by the NLHRS. 77 samples were found to be HCV Ab positive of which 74 individuals had been previously identified by the HCV nurse as having been exposed to the virus. Therefore, 3 new cases of exposure were identified through the community-wide screening; RNA testing of DBS samples showed 12 RNA positive samples, 10 of which were of the 74 previously known cases, and 2 of which were of the 3 new cases. All new cases were linked to care.
Discussion
Previous to our 2017 pilot trial in Community 1, we found no record of any community-wide testing for HCV in Canada. Here we demonstrate the effectiveness of close collaboration between tertiary care professionals and local First Nations governance and the effective pioneering of DBS in Canada for community-wide HCV testing and linkage to care.
The very high turnout in both communities shows the power of locally-developed, specific community health care initiatives when endorsed by chief and council, advertised widely and appropriately, and accompanied by simple incentives. In both communities, a wide range of the population participated, with similar numbers of men and women, across all age groups. There was also no sign that participation was exhausted by the end of testing: in both communities testing remained steady, from day to day in Lac Seul, and from week to week in KI, suggesting that an expansion of the programs would have drawn even more people.
The use of DBS cards was successful, in that it allowed for great flexibility in the staffing of the program and ease of processing and shipping of the samples. From the results of Lac Seul, where the program was piloted, DBS sampling was not only successful, but of remarkably high quality, according to the receiving lab.
Limitations of the program include delays in the delivery of the DBS cards from Community 2 to the national testing lab in Winnipeg. Future endeavours should either prioritize rapid delivery of the samples to the testing lab or ensure the DBS cards are frozen immediately after they are dried, as that is known to improve sample integrity (13).
It is notable that the prevalence among those tested in Community 1 was very low (0.40%) whereas, in Community 2, 77 of 470 results (16.4%) were positive. The difference in HCV burden may reflect differences in the communities both in terms of the burden of injection drug use but also whether HCV has recently been introduced into the community. The results from Community 2 highlight that once introduced, HCV can spread widely through a small population. However, even in Community 2, very few people who had not already been tested by the HCV nurse were found to test HCV Ab positive. While this finding may suggest that the program was not successful, part of the goal was to determine whether HCV had spread into the community-at-large or was confined to those participating in the buprenorphine/naloxone program. Although community-wide testing in KI identified few new cases, this approach also normalizes testing thereby reducing stigma associated with the test, which may encourage testing by those at risk and those who may not be aware of their risk.
Indeed, it is possible that those with known risk may have avoided testing through the testing efforts because of fear of stigma should they be found to be infected with HCV. Strategies to reach those who did not participate are clearly important. Notably, the majority of people approached at their homes in the third week of testing at Community 2 were willing to participate; by this time knowledge of the program was widespread. Normalizing the testing of HCV and highlighting its curable nature have been shown to reduce stigma. Broad testing is also likely to make population testing approaches more acceptable in the dozens of other FN communities of northwest Ontario; the majority of which do not have a dedicated HCV nurse to drive other testing approaches. Whether testing should be population-wide or focused on specific groups may vary by community, both in terms of prevalence and most importantly in terms of preference of community members.
Sample handling was an issue in KI, an unexpected and likely avoidable issue. If DBS samples reach the lab promptly, false positive results are extremely rare (13). Prior to initiation of future testing efforts in the region, new strategies will have to be developed with the lab and public health authorities to ensure samples reach the lab in a timely manner. These challenges should be surmountable with proper planning. In each site the cost of the entire project was comparable to the price of a single treatment for HCV. For the cost, hundreds of people in an endemic region for HCV have been tested and have been educated about the virus through this program.
The program highlighted the importance of site champions. In both communities, active, enthusiastic involvement of community members and leaders was likely very important to the success of the programs. Identifying and engaging champions in other sites may be critical to the success of future programs. Notably, as illustrated by the success in Community 1, that person does not need to be an expert in HCV.
It is hoped this initiative challenges prevailing ideas of what can be achieved in remote communities in Ontario. Given the chance, both of these communities embraced the opportunity to meaningfully address this health care issue. The attitude of the health director in Community 1 was that ‘We need education, and awareness. A lot of people don’t know what the disease entails’ (Personal correspondence). The health director in Community 2 urged, ‘It doesn’t matter what it takes, let’s just get this fixed’ (Personal correspondence). The voices of these health directors, plus the high numbers of participants who engaged with program, reinforce that HCV can be effectively addressed when programs are developed in collaboration with community members.
Finally, it should be noted that despite the passage of a year which included the distractions and disruptions of COVID-19, the awareness in Community 2 regarding HCV, rose from level 3 before the intervention to level 4, suggesting the community-wide testing had a lasting impact on public perception about HCV.
The experience in both communities clearly indicates that broad HCV screening can be achieved even in rural and remote communities with limited health care infrastructure. Expansion of this type of program to employ innovative strategies for testing and engagement in care that are developed and led by community members, including First Nations and other Indigenous communities, will be required if Canada is to reach the WHO HCV elimination targets.
acknowledgments:
The authors would like to acknowledge the contribution of Chiefs Donny Morris and Clifford Bull and Health Director Shane Cutfeet to this work, as well as Aaron Vanderhoff for help with statistical analysis.
Ethics Approval:
The study been reviewed by the Sioux Lookout Meno Ya Win Research Review and Ethics Committee.
Informed Consent:
N/A
Registry and the Registration No. of the Study/Trial:
N/A
Funding:
This research was supported by a Canadian Liver Foundation research operating grant awarded in 2016 and Gilead Sciences.
Disclosures:
The authors have nothing to disclose.
Peer Review:
This manuscript has been peer reviewed.
Animal Studies:
N/A
Funding Statement
This research was supported by a Canadian Liver Foundation research operating grant awarded in 2016 and Gilead Sciences.
References
- 1.Kwong JC, Ratnasingham S, Campitelli MA, et al. The Impact of Infection on Population Health: Results of the Ontario Burden of Infectious Diseases Study. PLoS One 2012; 7:e44103. 10.1371/journal.pone.0044103. Medline: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lapointe-Shaw L, Chung H, Sander B, et al. Peri-complication diagnosis of hepatitis C infection: Risk factors and trends over time. Liver Int 2021; 41:33–47. Available at: https://onlinelibrary.wiley.com/doi/10.1111/liv.14670. 4 March 2021. 10.1111/liv.14670. Medline: [DOI] [PubMed] [Google Scholar]
- 3.Alter MJ. Epidemiology of viral hepatitis and HIV co-infection. J Hepatol 2006; 44:S6–9. Available at: http://www.ncbi.nlm.nih.gov/pubmed/16352363. 5 March 2021. 10.1016/j.jhep.2005.11.004. Medline: [DOI] [PubMed] [Google Scholar]
- 4.Popping S, Bade D, Boucher C, et al. The global campaign to eliminate HBV and HCV infection: International Viral Hepatitis Elimination Meeting and core indicators for development towards the 2030 elimination goals. J virus Erad 2019; 5:60–66. Available at: http://www.ncbi.nlm.nih.gov/pubmed/30800429. 2 June 2020. 10.1016/S2055-6640(20)30281-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.2nd World Indigenous Peoples’ Conference on Viral Hepatitis—Anchorage Consensus Statement 2017 | ATSIHIV. 2017. Available at: https://www.atsihiv.org.au/2nd-world-indigenous-peoples-conference-on-viral-hepatitis-anchorage-consensus-statement-2017/. 2 June 2020.
- 6.Wu H-X, Wu J, Wong T, et al. Incidence and risk factors for newly acquired hepatitis C virus infection among Aboriginal versus non-Aboriginal Canadians in six regions, 1999–2004. Eur J Clin Microbiol Infect Dis 2007; 26:167–174. Available at: http://www.ncbi.nlm.nih.gov/pubmed/17308895. 13 March 2018. 10.1007/s10096-007-0267-7. Medline: [DOI] [PubMed] [Google Scholar]
- 7.Nation Nishnawbe Aski. Resolution: 09/92. Prescription drug abuse state of emergency. Thunder Bay, ON: Nishnawbe Aski Nation; 2009. [Google Scholar]
- 8.Kanate D, Folk D, Cirone S, et al. Community-wide measures of wellness in a remote First Nations community experiencing opioid dependence: Evaluating outpatient buprenorphine-naloxone substitution therapy in the context of a First Nations healing program. Can Fam physician Médecin Fam Can 2015; 61:160–5. Available at: http://www.pubmedcentral.nih.gov/articlerender.fcgi?artid=4325865 &tool=pmcentrez&rendertype=abstract. 22 November 2015. [PMC free article] [PubMed] [Google Scholar]
- 9.Katt M, Chase C, Samokhvalov A, Argento E, Rehm J, Fischer B. View of feasibility and outcomes of a community-based taper-to-low-dose-maintenance suboxone treatment program for prescription opioid dependence in a remote First Nations community in Northern Ontario | Int J of Indig Health. 2012. Available at: https://jps.library.utoronto.ca/index.php/ijih/article/view/29021/23838. 28 April 2020.
- 10.Gordon J, Bocking N, Pouteau K, Farrell T, Ryan G, Kelly L. First Nations hepatitis C virus infections. Can Fam Physician 2017; 63. [PMC free article] [PubMed] [Google Scholar]
- 11.Fayed ST, King A, King M, et al. In the eyes of Indigenous people in Canada: Exposing the underlying colonial etiology of hepatitis C and the imperative for trauma-informed care. Can Liver J 2018; 1:115–129. Available at: https://canlivj.utpjournals.press/doi/10.3138/canlivj.2018-0009. 21 October 2021. 10.3138/canlivj.2018-0009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Assessing Community Readiness – Manual | CAAN. Available at: https://caan.ca/en/assessing-community-readiness/harm-reduction-implementation-guide/. 28 April 2020.
- 13.Lange B, Cohn J, Roberts T, Camp J, Chauffour J, et al. Diagnostic accuracy of serological diagnosis of hepatitis C and B using dried blood spot samples (DBS): two systematic reviews and meta-analyses. BMC Infect Dis. November 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]


