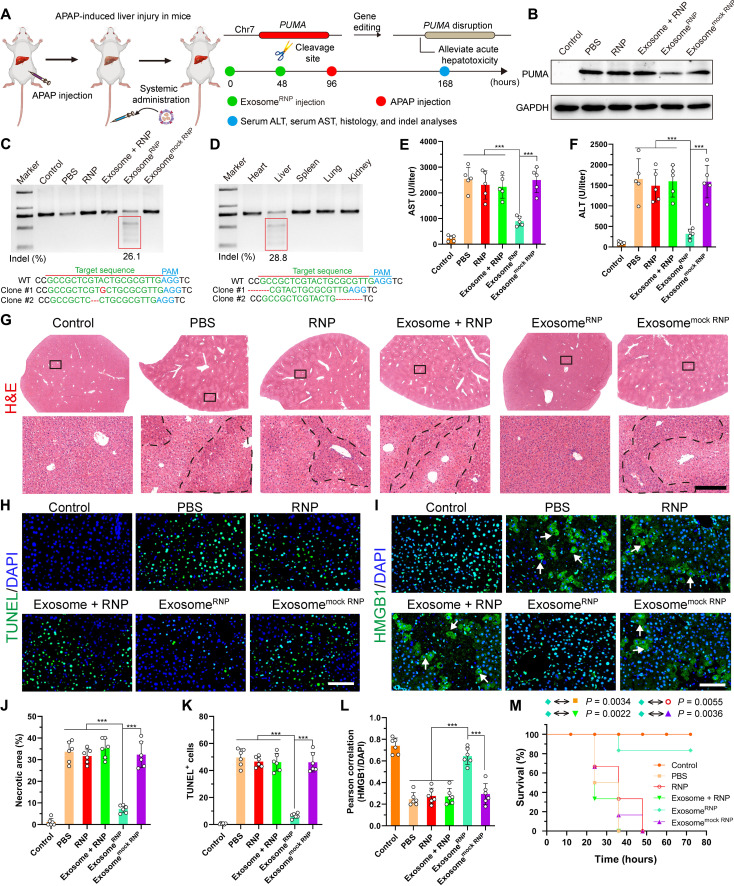
Fig. 3. ExosomeRNP-mediated genome editing suppressed APAP-induced acute liver injury and lethality.
(A) Schematic illustration of exosome for in vivo delivery of Cas9 RNP for the treatment of APAP-induced liver injury. APAP was administrated through intraperitoneal injection, and exosome/RNP complexes were administered through tail vein. (B) Western blotting of PUMA in livers of mice after the specified treatments. (C) Frequency of indel mutation detected by T7E1 assay from liver tissue after the specified treatments. (D) Frequency of indel mutation detected by T7E1 assay from different organs after exosomeRNP treatment at day 7. (E and F) Serum AST (E) and ALT (F) levels in mice after the specified treatments. (G) Hematoxylin and eosin (H&E) staining of liver sections from mice after the specified treatments. Representative images with dotted line indicating example necrotic centrilobular areas. Scale bar, 200 μm. The regions within the dotted lines denote the accumulation of blood cells. (H) TUNEL (green) staining of liver sections after the specified treatments. Scale bar, 80 μm. (I) HMGB1 (green) staining of liver sections after the specified treatments. Representative images with arrows indicating example cells with cytoplasmic HMGB1 staining and hollow nuclei. Scale bar, 80 μm. (J) Quantification of necrotic areas by ImageJ software. (K) Quantification of TUNEL signals by ImageJ software. (L) Quantification of colocalization of HMGB1 and nuclei by ImageJ software. Means ± SD; n = 6 [one-way analysis of variance (ANOVA) with a Tukey’s post hoc test, ***P < 0.001]. (M) Survival rates of mice after the specified treatments. Statistical significance was calculated by log-rank test (means ± SD, n = 6).