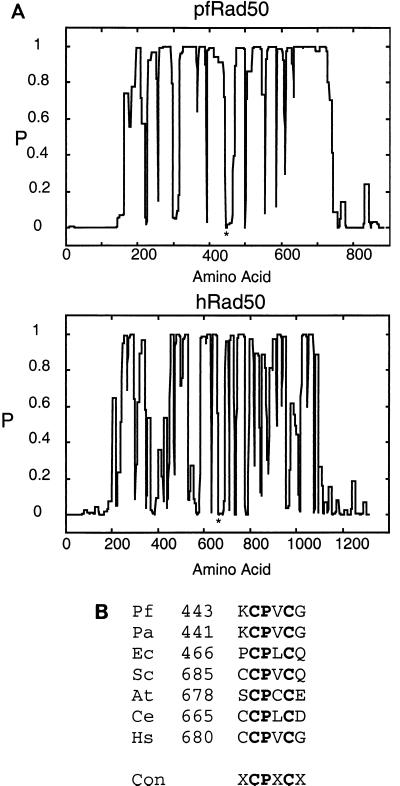
FIG. 2.
Structural conservation of pfRad50 and hRad50. (A) The human and P. furiosus Rad50 homologues were examined for the potential to form alpha-helical coiled-coil structures using the program COILS (see Materials and Methods). The plot shows the probability (P) of a given amino acid residue being present in an alpha-helical coiled-coil domain and indicates that the overall structure of the two proteins is conserved. The asterisk indicates the position of a conserved CPXC motif in each protein. (B) Sequence comparison of a number of Rad50 homologues shows the presence of a conserved (Con) CPXC motif in the central portion of each molecule. This region of the molecule is in a position that has a low probability of forming a coiled-coil structure (A), implicating it as a potential hinge region in the coiled-coil domain. Organisms listed are P. furiosus (Pf), Pyrococcus abyssi (Pa), E. coli (Ec), S. cerevisiae (Sc), Arabidopsis thaliana (At), Caenorhabditis elegans (Ce), and Homo sapiens (Hs).