FIG. 4.
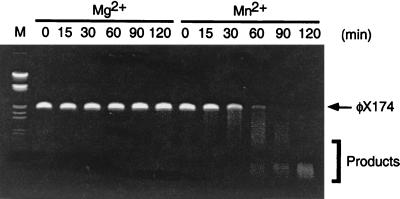
Single-strand endonuclease activity of pfMre11. Based on the presence of the conserved nuclease domains in the N terminus of pfMre11, we examined the pfMre11 complex for nuclease activity. Purified protein was incubated with φX174 single-stranded DNA, and the extent of single-strand endonuclease activity was assessed by gel electrophoresis. The reactions were carried out in the presence of either 5 mM MgCl2 (Mg2+) or 5 mM MnCl2 (Mn2+). The results indicate that pfMre11 is a Mn2+-dependent single-strand endonuclease. Identical results were obtained with pfMR50 (data not shown).