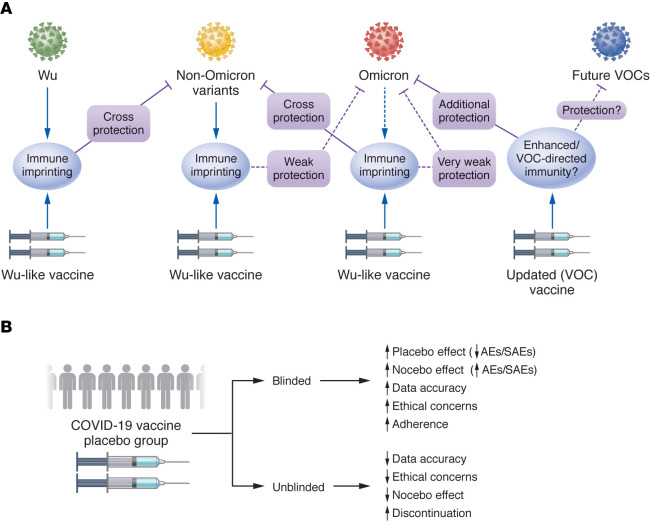
Figure 1. Lessons and questions from a large-scale COVID-19 vaccine trial.
(A) Immune imprinting and durability of protection. The immunogenic components in SARS-CoV-2 vaccines are derived from early (2019 to 2020) WT or Wuhan-like (Wu-like) viral isolates. Vaccine-stimulated immunity results in antibodies that neutralize infection by homologous or closely related viral strains (solid lines), but does not confer long-lasting protection. Immune imprinting from either vaccination or infection with early SARS-CoV-2 strains means that subsequent infections with newer SARS-CoV-2 variants stimulate existing memory cells specific for previously encountered SARS-CoV-2 variants, rather than recruiting additional immune cells better suited to the new variants. Therefore, the antibodies produced may not efficiently neutralize subsequent variants (dashed lines). Over time, protection and cross-protection are further diminished due to declining antibody levels. The Omicron variant and emerging lineages bear numerous immune escape mutations and are poorly neutralized by antisera specific for older SARS-CoV-2 strains. The durability and breadth of immune protection may be enhanced with booster dose vaccination and updated vaccines. (B) Trade-offs of placebo controls. Placebo control blinding and unblinding are a trade-off between the accuracy of RCTs’ primary and secondary outcome measures and ethical/social justice concerns.