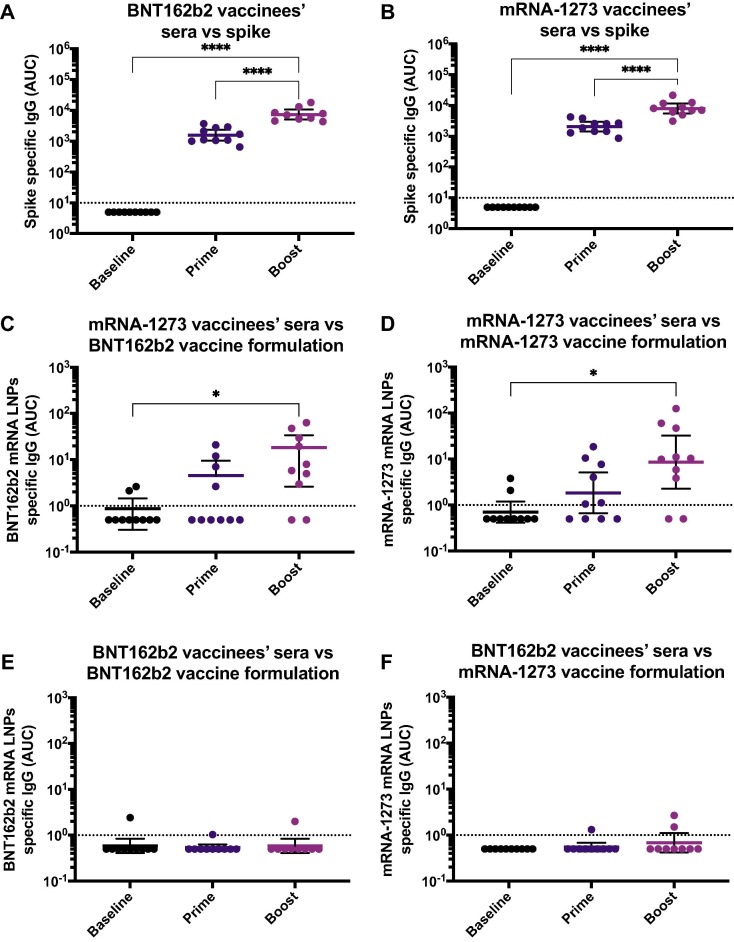
Fig. 1.
Antibodies against SARS-CoV-2 spike and mRNA-based vaccine formulations in vaccinees’ sera. Sera from BNT162b2 (left column) or mRNA-1273 (right column) vaccinees collected at baseline (n = 10 for BNT162b2 and n = 10 for mRNA-1273 groups), 18.9 days (arithmetic mean ± 2.4 SD) after the prime (n = 10 for BNT162b2 and n = 10 for mRNA-1273 groups) and 19.3 days (arithmetic mean ± 3.9 SD) after the boost (n = 10 for BNT162b2 and n = 10 for mRNA-1273 groups), were tested for IgG antibodies against SARS-CoV-2 full-length spike (A and B), BNT162b2 mRNA LNPs (C and E), and mRNA-1273 mRNA LNPs (D and F) by ELISA. Dotted line represents the limit of detection (LOD) of the assay. Statistically significant differences between post-prime/post-boost vs baseline antibody levels are shown. One-way ANOVA with Tukey’s multiple comparisons test. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.