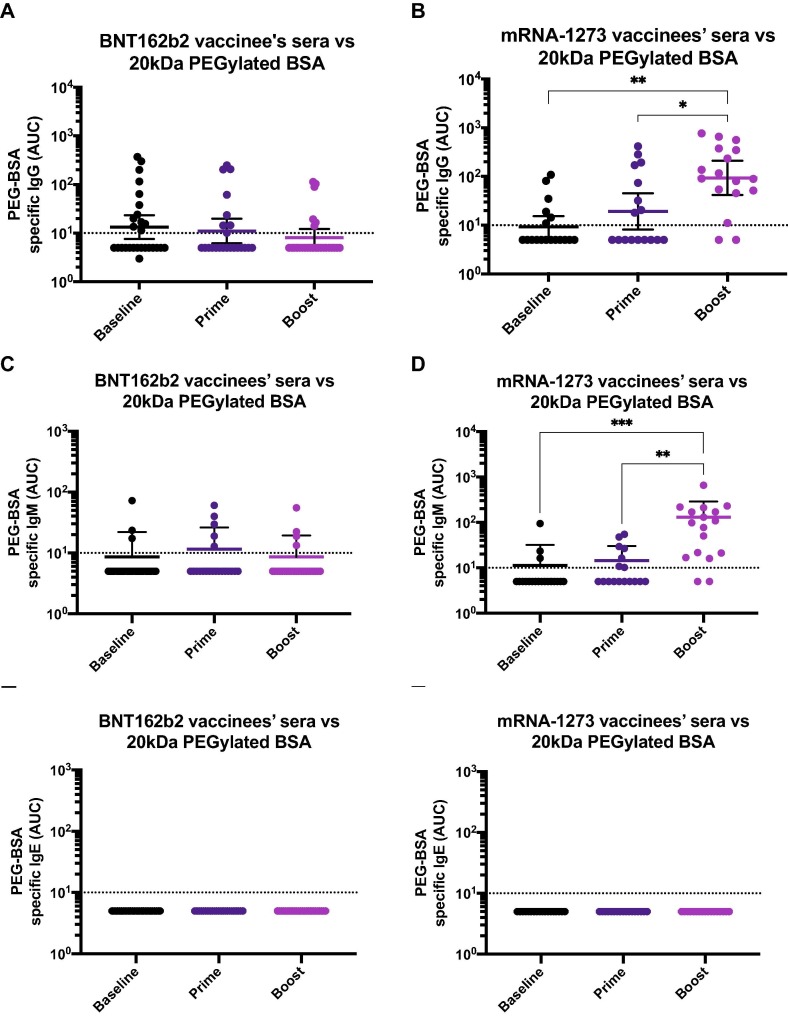
Fig. 5.
Polyethylene glycol (PEG) antibodies in participants selected based on their vaccine associated side effects including delayed onset injection site reactions and severe allergic reaction. Sera from BNT162b2 (A) or mRNA-1273 (B) vaccinees was collected at baseline (n = 28 for A and C, and n = 19 for B and D), 14.5 days (arithmetic mean ± 4.8 SD) after the prime (n = 23 for A and C, and n = 17 for B and D) or 25.2 days (arithmetic mean ± 11.6 SD) after the boost (n = 26 for A and C and n = 17 for B and D), and tested for IgG (A and B), IgM (C and D) or IgE (E and F) antibodies against 20 kDa PEGylated BSA by ELISA. Dotted line represents the limit of detection (LoD) of the assay. Statistically significant differences between post-prime/post-boost vs baseline antibody levels are shown. One-way ANOVA with Tukey’s multiple comparisons test was used to determine statistical significance. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001.