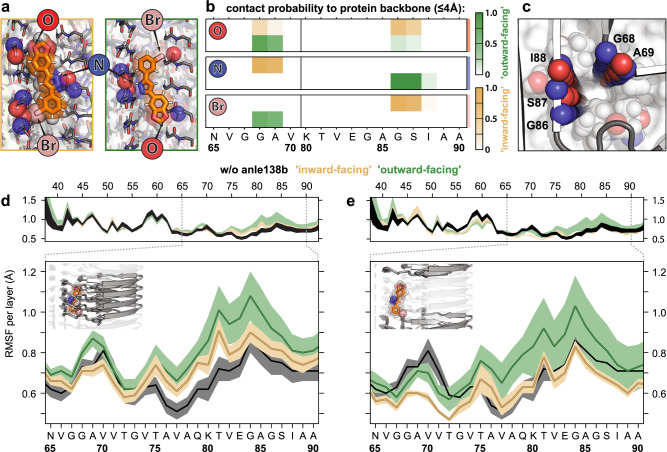
Fig. 4. Anle138b forms polar interactions with the protein backbone that alter local structural fluctuations in the protofilament.
a Polar contacts between anle138b atoms (indicated by arrows) and protein backbone atoms (blue circles—amide nitrogen; red circles—carbonyl oxygen) observed in MD simulations for inward- (yellow) and outward-facing (green) internal binding poses. b Contact probabilities for polar interactions with backbone atoms of individual residues for polar interactions defined in a. Scale bars (right) indicate contact probabilities. c Tubular cavity viewed up close down the long axis of the α-synuclein protofilament (shown without anle138b for clarity). Prominent contact sites in the backbone scaffold are represented by circles (colors as in a). d, e Average root mean squared fluctuations (RMSF) for d the four consecutive β-strands in contact with anle138b and e β-strands without close polar interactions. Average RMSF without anle138b present shown for comparison (black lines). β-strands of the fibril ends were not considered. Data are presented as mean values ± SEM (depicted by shading). Source data are provided as a Source Data file.