Abstract
The U.S. Department of Agriculture (USDA) regulates the potency testing of leptospirosis vaccines, which are administered to animals to protect against infection by Leptospira bacteria. Despite the long-term availability of in vitro test methods for assessing batch potency, the use of hamsters in lethal in vivo batch potency testing persists to varying degrees across leptospirosis vaccine manufacturers. For all manufacturers of these products, data collected from public USDA records show an estimated 40 percent decline in the annual use of hamsters from 2014 to 2020, with an estimated 55 percent decrease in the number of hamsters expected to have been used in leptospirosis vaccine potency tests (i.e., those in USDA Category E). An estimated 49,000 hamsters were used in 2020, with about 15,000 hamsters in Category E specifically. Based on this assessment, additional efforts are needed to fully implement in vitro batch potency testing as a replacement for the in vivo batch potency test. We propose steps that can be taken collaboratively by the USDA Center for Veterinary Biologics (CVB), manufacturers of leptospirosis vaccines, government agencies, and non-governmental organizations to accelerate broader use of the in vitro approach.
Keywords: Leptospira vaccines, veterinary vaccines, alternative methods, in vitro methods, replacement, potency testing
1. Introduction
In 2012, a workshop co-organized by the National Toxicology Program (NTP) Interagency Center for the Evaluation of Alternative Toxicological Methods (NICEATM), the U.S. Department of Agriculture (USDA) Center for Veterinary Biologics (CVB), the International Alliance for Biological Standardization (IABS), and other partner organizations sought to develop an internationally-relevant strategy to replace the use of animals in tests that are required during the assessment of veterinary leptospirosis vaccines. Participants gave special attention to the barriers remaining to replace one test in particular: the in vivo vaccine potency test using hamsters. This in vivo test was introduced as a CVB standard requirement for evaluating leptospirosis vaccines in 1974 and has remained functionally unchanged since that time, even though the currently-available in vitro replacements with CVB-standardized protocols and reference standards have been available since 2006, following refinement based on feedback from manufacturers and internal testing by CVB after their initial publication in 2000 [1,2]. At the time of the 2012 workshop, six years after CVB had approved the use of in vitro potency tests for vaccines targeting four leptospiral serovars, the in vitro methods were not widely used as replacements for the in vivo potency test. Workshop participants agreed on a number of strategies to complete implementation of the in vitro potency test and shared their findings in a series of reports published in Biologicals in 2013. This paper reviews the progress made in the U.S. to reduce the use of hamsters in leptospirosis vaccine batch potency testing since the 2012 workshop and the remaining obstacles to replace the use of hamsters with CVB’s currently available in vitro methods.
Leptospirosis is a zoonotic disease caused by the bacteria Leptospira. The disease, which can occur in many mammalian species, is spread through soil and water that has been contaminated by urine from infected animals [3]. Signs of infection vary by species. In humans, leptospirosis can cause fever, meningitis, internal bleeding, organ failure, and death [4]. In other animals, leptospirosis can cause lethargy, vomiting, diarrhea, organ failure, fetal abortion or stillbirth (in pregnant animals), and death [5,6]. There are no human vaccines made for leptospirosis in the U.S., though veterinary vaccines are used to avoid the spread of the disease to humans in addition to preventing illness among vaccinated animal populations [2]. In the U.S., leptospirosis vaccines are available for cows, pigs, and dogs, offering protection against the following leptospirosis serovars: Leptospira interrogans serogroups pomona, icterohaemorrhagiae, and canicola; Leptospira kirschneri serogroup grippotyphosa; and Leptospira interrogans serogroup hardjo and/or Leptospira borgpetersenii serogroup hardjo [1,3,7].
Regulatory guidelines require that leptospirosis vaccines are evaluated for purity, potency, safety, and efficacy. The USDA CVB regulates the batch potency testing of leptospirosis vaccines through the standard requirements for inactivated bacterial products codified in Title 9 of the Code of Federal Regulations (CFR 113.101–105) [7].
2. USDA requirements for leptospirosis vaccine potency testing
The batch potency test described in CVB regulations for leptospirosis is an in vivo challenge test conducted in hamsters. This 14-day test conducted on each batch of vaccine requires a minimum of 20 hamsters: 10 vaccinated and 10 control hamsters (Figure 1).
Figure 1.
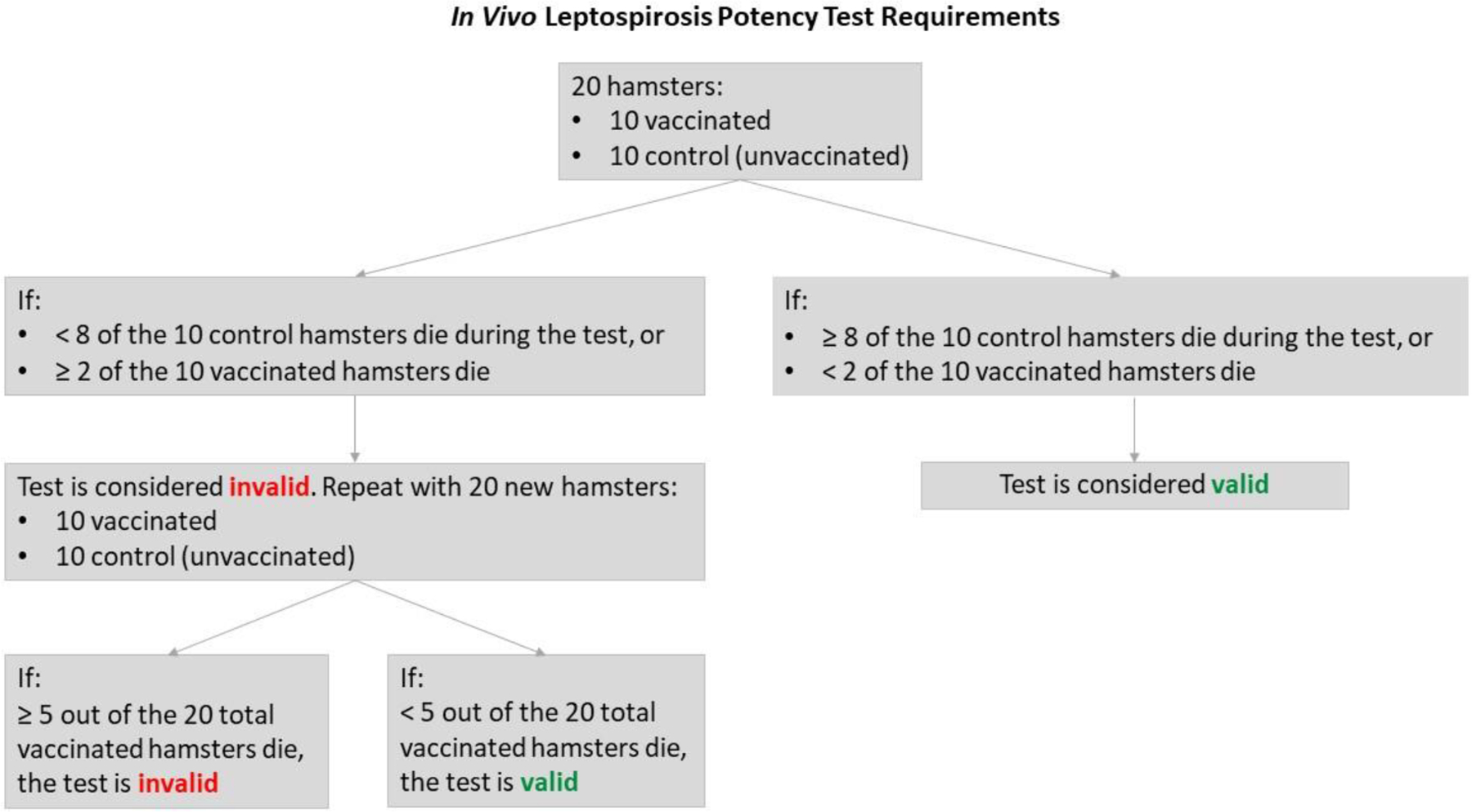
Flow chart describing USDA CVB in vivo potency test requirements for leptospirosis vaccines
In addition, the requirements stipulate a back-titration test that uses 20 more hamsters to calculate the lethal dose 50 (LD50) of a given vaccine batch, bringing the total number of hamsters used to at least 40, including the 20 hamsters needed for the principal in vivo test [7–9]. According to the CFR, a potency test must be conducted for each antigenic fraction associated with a label claim [10]. As all of the currently produced combination leptospirosis vaccine products contain at least four regulated leptospiral serogroups found in the U.S., approximately 160 hamsters may be used to test a single batch of one combination vaccine [2]. Because of the many steps involved, in vivo testing can take months—the in vivo test process involves acclimating the hamsters to the test facility, vaccinating the hamsters, carrying out and completing the challenge test, preparing the final test packet and quality review of the test, and repeating prior steps as necessary if the test is deemed invalid and repeated [1].
Serogroup-specific in vitro potency assays are available for four of the regulated serogroups of leptospirosis: Leptospira interrogans serogroups pomona, icterohaemorrhagiae, and canicola and Leptospira kirschneri serogroup grippotyphosa. These in vitro assays can replace the in vivo hamster test and are described in USDA CVB Supplemental Assay Methods (SAMs) 624, 625, 626, and 627[11–14]. Each of these SAMs describes a sandwich enzyme-linked immunosorbent assay (ELISA) that measures the relative potency of serovar-specific bacterins (killed or attenuated bacteria used to manufacture the vaccines) in comparison to a reference standard[1]. In this sandwich ELISA potency assay, a rabbit polyclonal antibody serves as the capture antibody and a mouse monoclonal antibody is the detecting antibody [11–15]. CVB notes that each of these in vitro methods is a “reproducible, sensitive, specific, and inexpensive alternative” to the in vivo test and vaccine manufacturers may also propose modifications to the SAM protocol to better suit their specific products [2,10].
Performing one of the in vitro SAMs takes one to three days, depending on whether manufacturers perform any optimization steps for their specific products, if they vary their incubation times for the antibody capture step, or if they include an additional elution step, which involves separating an antigen from an adjuvant and may add a day or more to the assay procedure (personal communication with CVB) [11–14]. While the in vivo test uses live, virulent Leptospira bacteria that could potentially harm the human technicians carrying out the test, the in vitro test does not [15]. The in vitro method has been shown to be equal to the in vivo test at assessing potency, is less time-consuming (completed in days instead of weeks), costs approximately 50 percent less than the in vivo test, and does not use live hamsters [16].
3. Workshop on alternatives for leptospira testing held in 2012
Alternatives to in vivo leptospirosis vaccine potency testing were discussed at the 2012 workshop [16]. Key workshop recommendations included but were not limited to CVB evaluating the necessity of the back-titration test requirement, companies achieving product-specific validation of the in vitro ELISA assay, CVB sharing data from the validation studies supporting the use of standard reference bacterins to assist manufacturers in their product-specific validation of the SAMs, and companies implementing the use of euthanasia and anesthesia to reduce suffering for hamsters when the in vivo test is still conducted. Notably, since the workshop, CVB introduced two back-titration waivers in 2015 and 2016, CVB Notice Nos. 15–13 and 17–06, which can reduce by half the total number of hamsters used in batch potency testing [8,9]. The other recommendations are either still underway or have not been publicly discussed since the workshop.
4. Method of Analysis
4.1. Hamster use trends for the potency test
Data on hamster use were gathered from the USDA’s Animal and Plant Health Inspection Service (APHIS) Public Search Tool, which includes annual reports of animal use numbers from facilities using animals included in the Animal Welfare Act [17]. The number of hamsters used between 2014 and 2020 were compiled for companies with active leptospirosis product licenses in one or more of those years as well as the number of hamsters used by CVB, which manufactures components used in in vivo leptospirosis potency tests that are derived from live hamsters (Table 1 and Table 2). Additional public reports published by APHIS were used, including “Veterinary Biological Products: Licensees and Permittees” (April 2020) and “Veterinary Biological Products in Licensed Establishments: Produced and Destroyed” (2016–2020). Previous installments of these documents (“Veterinary Biological Products: Licensees and Permittees” from 2012–2019 and “Veterinary Biological Products in Licensed Establishments: Produced and Destroyed” from 2012–2015) were also gathered through a Freedom of Information Act (FOIA) request to APHIS. Companies included in Tables 1 and 2 had active licenses for leptospirosis vaccine products in the years that are marked with asterisks (*), indicating that the hamsters reported in those years are likely to have been used in leptospirosis potency testing. The licensee reports are typically issued quarterly by the USDA, and as the most recent licensee report is from April 2020, quarterly reports from April of each year (2014 to 2019) were used to indicate which companies had active leptospirosis product licenses in each year.
Table 1.
Overall hamster use in the U.S. by company from 2014–2020. Asterisks (*) indicate companies that had active leptospirosis vaccine product licenses in that year, as per public reports. The symbols correspond to companies that merged, were acquired, or otherwise modified how animal use was reported to the USDA by company name.
| Annual Hamster Use in the U.S. by Company | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Merial Inc.† | Boehringer Ingelheim Animal Healtht† | Novartis Animal Health§ | Elanco Animal Health§ | Zoetis Inc.¶ | Zoetis LLC¶ | Colorado Serum Company | Diamond Animal Health | Intervet Inc. (Merck Animal Health) | USDA CVB | TOTAL Overall | |
| 2020 | 0 | 11966* | 0 | 11368* | 0 | 16931* | 921* | 3005* | 4747* | 78 | 49016 |
| 2019 | 1670* | 8552* | 0 | 14294* | 0 | 20285* | 148* | 1992* | 4691* | 92 | 51724 |
| 2018 | 5050* | 2797* | 0 | 19625* | 0 | 22099* | 167* | 1192* | 4820* | 571 | 56321 |
| 2017 | 5210* | 3399* | 0 | 17593* | 0 | 23692* | 594* | 6308* | 5626* | 3004 | 65426 |
| 2016 | 4935* | 15484* | 0 | 9071* | 9516* | 19625* | 401* | 6314* | 5886* | 3104 | 74336 |
| 2015 | 4285* | 14250* | 3699* | 4988 | 26433* | 0 | 1348* | 3759* | 7406* | 2689 | 68857 |
| 2014 | 6520* | 18660* | 8617* | 0 | 28817* | 0 | 782* | 5765* | 8190* | 2595 | 79946 |
Merial Inc. merged with Boehringer Ingelheim Animal Health in 2017.
Elanco Animal Health acquired Novartis Animal Health in 2014.
Zoetis LLC operated as Zoetis Inc. through 2016.
Table 2.
The number of hamsters used in Category E testing in the U.S. from 2014 to 2020. Asterisks (*) indicate companies that had active leptospirosis vaccine product licenses in that year, as per public reports. The symbols correspond to companies that merged, were acquired, or otherwise modified how animal use was reported to the USDA by company name.
| Category E Only: Annual Hamster Use in the U.S. by Company | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Merial Inc.† | Boehringer Ingelheim Animal Healtht† | Novartis Animal Health§ | Elanco Animal Health§ | Zoetis Inc.¶ | Zoetis LLC¶ | Colorado Serum Company | Diamond Animal Health | Intervet Inc. (Merck Animal Health) | USDA CVB | TOTAL Cat E | |
| 2020 | 0 | 6118* | 0 | 3461* | 0 | 3803* | 253* | 828* | 966* | 10 | 15439 |
| 2019 | 635* | 6231* | 0 | 4831* | 0 | 4502* | 56* | 549* | 696* | 25 | 17525 |
| 2018 | 1770* | 2762* | 0 | 7563* | 0 | 4444* | 3* | 688* | 804* | 133 | 18167 |
| 2017 | 1870* | 3318* | 0 | 12450* | 0 | 4110* | 76* | 3448* | 1382* | 700 | 27354 |
| 2016 | 1796* | 15016* | 0 | 3028* | 1189* | 2999* | 74* | 3977* | 1157* | 998 | 30234 |
| 2015 | 1728* | 14007* | 1237* | 1894 | 3900* | 0 | 302* | 3759* | 1194* | 1027 | 29048 |
| 2014 | 2565* | 18633* | 2899* | 0 | 4001* | 0 | 187* | 3669* | 1413* | 803 | 34170 |
Merial Inc. merged with Boehringer Ingelheim Animal Health in 2017.
Elanco Animal Health acquired Novartis Animal Health in 2014.
Zoetis LLC operated as Zoetis Inc. through 2016.
Table 1 describes overall hamster use in the U.S. by year. It is possible that some of these hamsters may have been used for experiments not related to leptospirosis vaccines. Nevertheless, the trend shows a decrease from 79,946 hamsters used annually in testing in the U.S. in 2014 to 49,016 hamsters used in 2020. This is an approximately 40 percent decrease in hamster use overall from 2014 to 2020.
4.2. USDA categories for animal use
The USDA requires companies to report a publicly available accounting of their use of animals by species, for those species included in the Animal Welfare Act. When reported to the agency, these animals are categorized into one of four categories: Category B (animals bred or held for research/testing but have not yet been used), C (animals used in research/testing involving no pain or distress), D (animals used in research/testing involving pain or distress for which anesthesia, analgesia, or tranquilizers were used), or E (animals used in research/testing involving unrelieved pain or distress). For animals used in Category E procedures, companies must provide scientific justification for why pain and/or distress could not be relieved. Hamsters used in leptospirosis vaccine-challenge tests are counted in Category E. Although the written justification is not always complete in its description of which procedure or product is being assessed, the leptospirosis vaccine potency test is the only instance in which the use of hamsters is mandated in the standard requirements for these products outlined in 9 CFR 113.101–105. With these assumptions in mind, the number of Category E hamsters is likely a close estimate of the number of hamsters used each year in leptospirosis vaccine potency testing in the U.S.
Table 2 shows that the total number of hamsters used in Category E experiments decreased from 34,170 hamsters in 2014 to 15,439 hamsters used in 2020, reflecting a 55 percent decrease. Refer to Appendix A for the USDA Category B-E breakdowns per year from 2014 to 2020.
4.3. Leptospirosis vaccine product information
Vaccine product and dose data were gathered from the USDA APHIS Current CVB Notices and from information received through FOIA requests for public data held by the USDA. This information was used to compare annual counts of hamsters used to the number of leptospirosis vaccine products made. Public reports of the number of product doses manufactured each year only provide the number of each product made in instances where three or more companies produce products under the same code (as this information is otherwise considered proprietary).
The totals in Table 3 were provided by USDA’s APHIS program office through a FOIA request and include the total number of all Leptospira-containing vaccine and bacteriological products produced and destroyed in each year. This total number of leptospirosis product doses includes products made by all companies, but the breakdown by each product code or company is unknown based on the format in which this information is publicly reported.
Table 3.
The number of leptospirosis vaccine doses produced and destroyed annually in the U.S. from 2015–2020, as stated in USDA reports for Leptospira-containing products made by three or more companies. This includes all reported leptospirosis combination vaccine products produced each year by any companies. Note that due to record retention policies, USDA does not keep data for more than seven years. Because the reporting for 2014 only included August 26, 2014 to December 31, 2014, they are not included in this table.
| Annual Total Leptospirosis Product Doses | |||
|---|---|---|---|
| Total Produced | Total Destroyed | Net Total Doses | |
| 2020 | 220,444,080 | 4,337,508 | 216,106,572 |
| 2019 | 185,320,906 | 6,291,676 | 179,029,230 |
| 2018 | 224,341,120 | 8,384,110 | 215,957,010 |
| 2017 | 233,910,154 | 2,149,049 | 231,761,105 |
| 2016 | 228,063,268 | 3,342,475 | 224,720,793 |
| 2015 | 205,773,909 | 1,471,290 | 204,302,619 |
The total number of net doses of leptospirosis vaccines produced each year between 2015 and 2020 has remained about the same (Table 3), suggesting that the decrease in hamster use cannot be explained by a reduction in annual production of leptospirosis bacteriological and vaccine products. Appendix B includes the number of doses of each product produced and destroyed annually, including totals and for individual products, as reported when those products are made by three or more companies.
4.4. Leptospirosis serogroup hardjo
From 2015 to 2020, companies have manufactured combination leptospirosis vaccines containing five serogroups of Leptospira bacteria: Leptospira interrogans serogroups 1) pomona, 2) icterohaemorrhagiae, and 3) canicola; 4) Leptospira kirschneri serogroup grippotyphosa; and 5) Leptospira interrogans serogroup hardjo or Leptospira borgpetersenii serogroup hardjo. The requirements for testing serogroup hardjo, per 9 CFR 113.105, do not distinguish between the two types of hardjo. A specific potency test is not specified in the standard requirement for this product as is the case for the other four serovars [18]. Instead, manufacturers of serogroup hardjo vaccines must submit a proposed potency test of their choosing per 9 CFR 113.105(c). Serogroup hardjo is considered difficult to test in vivo because it is not lethal in hamsters [18]. At the time of this publication, no in vitro method for assessing the potency of vaccines directed against serogroup hardjo has been made publicly available.
According to 9 CFR 113.9, a new potency test will not be considered confidential information after three product licenses are issued for the product or if use of the test is authorized by the licensee. If either of these conditions is met, the potency test may be published as a Standard Requirement by the USDA.
5. Discussion and next steps
5.1. Reasons for reductions in hamster use
There are several potential reasons for the reduction in hamster use seen in Tables 1 and 2, including companies successfully waiving the back-titration step for in vivo testing and implementing the in vitro ELISA method. However, it is not possible to know the reasons for reduced hamster use or for continued hamster use based on public data alone. For example, as of 2018, six manufacturers received exemptions for 35 total leptospirosis vaccine products from back-titration testing requirements per CVB Notices 15–13 and 17–06. By 2021, all U.S. leptospirosis product manufacturers had filed back-titration waivers for 37 total products (personal communications with CVB).
Aside from the successful adoption of back titration waivers, vaccine manufacturer implementation of the in vitro potency test methods is the most significant step toward replacing the use of animals to assess leptospirosis vaccines. Yet as of 2019 (the most recent year for which this information is available) only four of six manufacturers have transitioned to using the in vitro test methods for 21 total vaccine products. None of these manufacturers were using the in vitro methods exclusively (personal communication with CVB).
5.2. Consistency approach
At the 2012 workshop, vaccine manufacturers shared their challenges in switching from the in vivo to the in vitro potency test [16]. One action item from this workshop was for vaccine manufacturers to continue product-specific validation of the in vitro tests and investigate parameters that indicate and ensure product manufacturing consistency [16]. The consistency approach asserts that rigorously controlled production conditions during the manufacture of iterative batches of a biological product can lessen the need to rely on batch testing to establish conformity to quality and safety metrics. Increasing the consistency of production, then, reduces the need for repetitive batch potency testing. The European Union Reference Laboratory for alternatives to animal testing (EURL ECVAM) organized international workshops in 2006 and 2010 to discuss the consistency approach and its potential to reduce the number of animals used in testing of biological products, and at the 2012 workshop, CVB was encouraged to take steps to help companies with this process. To do so, CVB was encouraged to share archival ELISA testing data with vaccine manufacturers and to provide monoclonal antibodies and reagents for manufacturers to perform the product-specific validation steps necessary to implement the in vitro methods [16]. In 2021, CVB reported that they had shared the archival information requested during the workshop, participated in group and individual discussions with companies about the in vitro methods, and continue to provide references and reagents needed to conduct the in vitro test (personal communication with CVB).
To further reduce animal use, CVB could enact a policy to maintain consistency of the production of batches of leptospirosis vaccine products. During a roundtable discussion at the 2012 workshop, it was recommended that manufacturers and regulatory agencies consider using the consistency approach to help achieve international harmonization of potency testing requirements [19].
5.3. Optimization of the in vitro potency test method
CVB makes available 17 reagents for use with leptospirosis vaccine testing: four for use in the in vivo potency test and 13 for use with the in vitro SAMs (Appendix C). These reagents are divided into four broad categories: challenge cultures for the in vivo test, reference bacterins for the in vitro ELISA tests, polyclonal antibodies for the in vitro ELISA tests, and monoclonal antibodies for the in vitro ELISA tests [20]. With the exception of the reference bacterins, all of the reagents used to evaluate the potency of these products are animal-derived. The challenge cultures are produced from liver tissue excised from hamsters infected with Leptospira strains, the polyclonal antibodies are isolated from the serum of New Zealand white rabbits hyperimmunized against leptospiral serovars, and the monoclonal antibodies are produced using mice implanted with antibody-secreting hybridomas via the ascites method. In 2021, most antibodies produced by CVB are made by culturing animal-derived hybridomas in bioreactor chambers and the ascites method is only used when the quality of antibodies produced using other means is determined by CVB to be inadequate for their applications. These ascites-derived antibodies were previously prepared and are currently stockpiled by CVB. In the future, CVB will make monoclonal antibodies for use with the in vitro leptospirosis vaccine potency test as needed from animal-derived hybridomas cultured in bioreactors. Additionally, the serum used in the in vitro SAMs, which is collected from immunized rabbits, is also stockpiled and is expected to last for a decade (personal communication with CVB).
As more manufacturers switch to the in vitro SAMs, there is an opportunity to replace the use of animal-derived components with non-animal reagents. In 2020, EURL ECVAM released a recommendation that animal-derived antibodies should not be used for research or regulatory purposes [21]. Replacement of animal-derived antibodies and other animal-derived reagents is important to maximize the reproducibility of testing, including in vitro leptospirosis potency testing. The polyclonal and monoclonal antibodies needed for the in vitro tests can be sequenced and made available as recombinant, animal-free antibodies. This switch could allow for a more accurate reading of vaccine potency, leading to more consistency in vaccine batches. In addition, recombinant antibodies can be produced with higher specificity and affinity in comparison to animal-derived antibodies, are more easily produced with identical affinity characteristics between batches, and have a faster generation time compared to animal-derived antibodies [22].
Manufacturers can submit proposals for modifications of already approved assays, including to request the use of recombinant antibodies in place of the current animal-derived reagents in the in vitro assay method (personal communication with CVB). While the stockpiles of existing leptospiral antibodies and antiserum may last for some years, there are numerous advantages to transitioning to recombinant antibodies immediately [23,24]. Therefore, transitioning to recombinant antibodies should be prioritized in place of using animal-derived antibodies to maximize the reproducibility of the in vitro assays and support their uptake.
5.4. Incentives for the use of in vitro methods
CVB could implement programs to help leptospirosis manufacturers more easily transition to in vitro potency testing by providing a formal framework for resolving questions and problems with adopting in vitro methods. This program could be comparable to the U.S. Food and Drug Administration’s (FDA’s) Medical Device Development Tools (MDDT) program and Innovative Science and Technology Approaches for New Drugs (ISTAND) pilot program, which are designed to expedite the agency’s processes for qualifying the use of new nonclinical toxicology and pharmacology test methods for medical devices and pharmaceuticals, respectively [25,26]. Through an approach based on these programs, vaccine manufacturers may be able to receive guidance and assistance more easily from CVB as they develop and test in vitro methods for their specific products—in collaboration with other manufacturers of similar products, if possible.
6. Conclusion
While the downward trend in hamster use shows that progress has been made, complete replacement of the in vivo potency test method has yet to be realized. Because the in vitro potency test has the potential to replace most of the routine use of hamsters for leptospirosis testing [16], a key next step is to systematically implement the use of the in vitro SAMs in place of the in vivo test. This will require collaborative partnerships that include CVB and vaccine manufacturers. Communication between stakeholders is needed to better understand the impact of the back-titration waivers and the in vitro ELISA method in reducing hamster use, and to identify the remaining hurdles preventing complete adoption of the ELISA method by all vaccine manufacturers. An expert meeting or targeted workshop would facilitate this discussion and highlight developments and challenges that have emerged since the 2012 NICEATM and CVB co-organized workshop. Additionally, while this paper focused on leptospirosis potency testing in the U.S., international harmonization is a critical next step. Given the global economy in which these vaccine manufacturers operate, implementing international regulatory use of the in vitro tests is the only way complete replacement of hamster use can be achieved.
Acknowledgments
The authors gratefully acknowledge the International Alliance for Biological Standardization (IABS) for reviewing the paper.
Appendices
Appendix A:
Category B-E annual hamster use by company. Note that the numbers reported for Zoetis LLC and Zoetis Inc. are combined into one table. All other companies/organizations listed in Tables 1 and 2 have separate tables below of the number of hamsters used in each category. In the final table below, titled “All Categories of Hamster Use Reported Annually for All Companies,” Category B hamsters (those being held for future use) are not included in the “Total Category E” and “Total Overall” columns (the overall column includes Category C – E); those totals correspond to the numbers reported in Tables 1 (overall) and 2 (Category E).
| Company: Merial Inc. | |||||
|---|---|---|---|---|---|
| Cat B | Cat C | Cat D | Cat E | Total used | |
| 2020 | 0 | 0 | 0 | 0 | 0 |
| 2019 | 0 | 912 | 123 | 635 | 1670 |
| 2018 | 0 | 2810 | 470 | 1770 | 5050 |
| 2017 | 0 | 2639 | 701 | 1870 | 5210 |
| 2016 | 0 | 2792 | 347 | 1796 | 4935 |
| 2015 | 0 | 1874 | 683 | 1728 | 4285 |
| 2014 | 0 | 3330 | 625 | 2565 | 6520 |
| Company: Boehringer Ingelheim Animal Health | |||||
|---|---|---|---|---|---|
| Cat B | Cat C | Cat D | Cat E | Total used | |
| 2020 | 608 | 5321 | 527 | 6118 | 11966 |
| 2019 | 496 | 2070 | 251 | 6231 | 8552 |
| 2018 | 132 | 35 | 0 | 2762 | 2797 |
| 2017 | 138 | 81 | 0 | 3318 | 3399 |
| 2016 | 90 | 323 | 145 | 15016 | 15484 |
| 2015 | 0 | 207 | 36 | 14007 | 14250 |
| 2014 | 389 | 13 | 14 | 18633 | 18660 |
| Company: Novartis Animal Health | |||||
|---|---|---|---|---|---|
| Cat B | Cat C | Cat D | Cat E | Total used | |
| 2020 | 0 | 0 | 0 | 0 | 0 |
| 2019 | 0 | 0 | 0 | 0 | 0 |
| 2018 | 0 | 0 | 0 | 0 | 0 |
| 2017 | 0 | 0 | 0 | 0 | 0 |
| 2016 | 0 | 0 | 0 | 0 | 0 |
| 2015 | 0 | 2462 | 0 | 1237 | 3699 |
| 2014 | 0 | 5718 | 0 | 2899 | 8617 |
| Company: Elanco Animal Health | |||||
|---|---|---|---|---|---|
| Cat B | Cat C | Cat D | Cat E | Total used | |
| 2020 | 261 | 7653 | 254 | 3461 | 11368 |
| 2019 | 376 | 9202 | 261 | 4831 | 14294 |
| 2018 | 1362 | 11810 | 252 | 7563 | 19625 |
| 2017 | 508 | 5064 | 79 | 12450 | 17593 |
| 2016 | 294 | 5961 | 82 | 3028 | 9071 |
| 2015 | 0 | 3094 | 0 | 1894 | 4988 |
| 2014 | 0 | 0 | 0 | 0 | 0 |
| Company: Zoetis LLC/Inc. | |||||
|---|---|---|---|---|---|
| Cat B | Cat C | Cat D | Cat E | Total used | |
| 2020 | 594 | 11991 | 1137 | 3803 | 16931 |
| 2019 | 53 | 14562 | 1221 | 4502 | 20285 |
| 2018 | 435 | 16334 | 1321 | 4444 | 22099 |
| 2017 | 551 | 18138 | 1444 | 4110 | 23692 |
| 2016 | 713 | 23139 | 1814 | 4188 | 29141 |
| 2015 | 721 | 20906 | 1627 | 3900 | 26433 |
| 2014 | 92 | 22791 | 2025 | 4001 | 28817 |
| Company: Colorado Serum Company | |||||
|---|---|---|---|---|---|
| Cat B | Cat C | Cat D | Cat E | Total used | |
| 2020 | 0 | 284 | 384 | 253 | 921 |
| 2019 | 0 | 0 | 92 | 56 | 148 |
| 2018 | 0 | 161 | 3 | 3 | 167 |
| 2017 | 7 | 394 | 124 | 76 | 594 |
| 2016 | 16 | 277 | 50 | 74 | 401 |
| 2015 | 177 | 877 | 169 | 302 | 1348 |
| 2014 | 165 | 504 | 91 | 187 | 782 |
| Company: Diamond Animal Health | |||||
|---|---|---|---|---|---|
| Cat B | Cat C | Cat D | Cat E | Total used | |
| 2020 | 0 | 2177 | 0 | 828 | 3005 |
| 2019 | 0 | 1443 | 0 | 549 | 1992 |
| 2018 | 0 | 504 | 0 | 688 | 1192 |
| 2017 | 0 | 2860 | 0 | 3448 | 6308 |
| 2016 | 0 | 2337 | 0 | 3977 | 6314 |
| 2015 | 1872 | 0 | 0 | 3759 | 3759 |
| 2014 | 0 | 2096 | 0 | 3669 | 5765 |
| Company: Intervet Inc. (Merck Animal Health) | |||||
|---|---|---|---|---|---|
| Cat B | Cat C | Cat D | Cat E | Total used | |
| 2020 | 410 | 2686 | 1095 | 966 | 4747 |
| 2019 | 183 | 2944 | 1051 | 696 | 4691 |
| 2018 | 198 | 2898 | 1118 | 804 | 4820 |
| 2017 | 511 | 3133 | 1111 | 1382 | 5626 |
| 2016 | 330 | 3430 | 1299 | 1157 | 5886 |
| 2015 | 122 | 4378 | 1834 | 1194 | 7406 |
| 2014 | 518 | 4626 | 2151 | 1413 | 8190 |
| Company: USDA CVB | |||||
|---|---|---|---|---|---|
| Cat B | Cat C | Cat D | Cat E | Total used | |
| 2020 | 0 | 61 | 7 | 10 | 78 |
| 2019 | 0 | 67 | 0 | 25 | 92 |
| 2018 | 0 | 424 | 14 | 133 | 571 |
| 2017 | 0 | 2213 | 91 | 700 | 3004 |
| 2016 | 0 | 1891 | 215 | 998 | 3104 |
| 2015 | 0 | 1529 | 133 | 1027 | 2689 |
| 2014 | 0 | 1669 | 123 | 803 | 2595 |
| All Categories of Hamster Use Reported Annually for All Companies | ||||||
|---|---|---|---|---|---|---|
| Cat B | Cat C | Cat D | Cat E | Total overall used (Cat C+D+E) | Total of all categories (B+C+D+E) | |
| 2020 | 1873 | 30173 | 3404 | 15439 | 49016 | 50889 |
| 2019 | 1108 | 31200 | 2999 | 17525 | 51724 | 52832 |
| 2018 | 2127 | 34976 | 3178 | 18167 | 56321 | 58448 |
| 2017 | 1715 | 34522 | 3550 | 27354 | 65426 | 67141 |
| 2016 | 1443 | 40150 | 3952 | 30234 | 74336 | 75779 |
| 2015 | 2892 | 35327 | 4482 | 29048 | 68857 | 71749 |
| 2014 | 1164 | 40747 | 5029 | 34170 | 79946 | 81110 |
Appendix B:
Leptospirosis vaccine products produced and destroyed annually by product, when data is reported for products made by three or more companies. Note that zeroes are included in the charts when they are reported; other values left blank are not reported (NR), but products could have been produced or destroyed by less than three companies and therefore would not be included.
| Doses of Leptospirosis Vaccine PRODUCED | |||||||
|---|---|---|---|---|---|---|---|
| Product Name | Product Code | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 |
| Leptospira Canicola-Grippotyphosa-Hardjo-Icterohaemorrhagiae-Pomona Bacterin | 266500 | NR | NR | NR | NR | 7,305,010 | 4,180,505 |
| Leptospira Canicola-Grippotyphosa-Hardjo-Icterohaemorrhagiae-Pomona Bacterin | 266501 | 7,354,920 | 8,253,280 | 10,200,090 | 7,593,700 | 7,953,960 | NR |
| Leptospira Canicola-Grippotyphosa-Hardjo-Icterohaemorrhagiae-Pomona Bacterin | 266502 | 12,478,915 | 15,986,405 | 16,414,360 | 17,865,045 | 14,661,995 | 14,515,685 |
| Leptospira Canicola-Grippotyphosa-Icterohaemorrhagiae-Pomona Bacterin | 266800 | 16,115,744 | 14,866,633 | NR | NR | NR | NR |
| Campylobacter Fetus-Leptospira Canicola-Grippotyphosa-Hardjo-Icterohaemorrhagiae-Pomona Bacterin | 286301 | 5,519,240 | 5,997,860 | 7,443,700 | 10,998,500 | 7,050,070 | 7,993,470 |
| Campylobacter Fetus-Leptospira Canicola-Grippotyphosa-Hardjo-Icterohaemorrhagiae-Pomona Bacterin | 286307 | NR | NR | NR | NR | 1,474,290 | NR |
| Leptospira Pomona bacterin | 269100 | NR | NR | NR | NR | NR | 1,628,900 |
| Totals for Bacterins & bacterial extracts | 78,283,860 | 85,690,255 | 86,466,858 | 88,979,541 | 83,623,243 | 76,675,652 | |
| Totals for Vaccines with bacterins/bacterial extracts/toxoids | 114,318,914 | 89,406,722 | 123,071,549 | 115,359,416 | 111,548,738 | 103,287,081 | |
| Totals for For further manufacture: bacterins & bacterial extracts | 27,762,131 | 10,133,669 | 12,212,208 | 28,150,227 | 25,299,977 | 15,670,486 | |
| Totals for For further manufacture: vaccines with bacterins/bacterial extracts/toxoids | NR | NR | 2,517,700 | 1,333,000 | 7,533,500 | 10,049,000 | |
| GRAND TOTAL | 220,444,080 | 185,320,906 | 224,341,120 | 233,910,154 | 228,063,268 | 205,773,909 | |
| Doses of Leptospirosis Vaccine DESTROYED | |||||||
| Product Name | Product Code | 2020 | 2019 | 2018 | 2017 | 2016 | 2015 |
| Leptospira Canicola-Grippotyphosa-Hardjo-Icterohaemorrhagiae-Pomona Bacterin | 266500 | NR | NR | NR | NR | 0 | 647,435 |
| Leptospira Canicola-Grippotyphosa-Hardjo-Icterohaemorrhagiae-Pomona Bacterin | 266501 | 376,900 | 0 | 469,100 | 0 | 552,500 | NR |
| Leptospira Canicola-Grippotyphosa-Hardjo-Icterohaemorrhagiae-Pomona Bacterin | 266502 | 0 | 0 | 0 | 0 | 0 | 0 |
| Leptospira Canicola-Grippotyphosa-Icterohaemorrhagiae-Pomona Bacterin | 266800 | 0 | 644,341 | NR | NR | NR | NR |
| Campylobacter Fetus-Leptospira Canicola-Grippotyphosa-Hardjo-Icterohaemorrhagiae-Pomona Bacterin | 286301 | 0 | 335,800 | 0 | 0 | 0 | 0 |
| Campylobacter Fetus-Leptospira Canicola-Grippotyphosa-Hardjo-Icterohaemorrhagiae-Pomona Bacterin | 286307 | NR | NR | NR | NR | 0 | NR |
| Leptospira Pomona bacterin | 269100 | NR | NR | NR | NR | NR | 0 |
| Totals for Bacterins & bacterial extracts | 376,900 | 3,774,478 | 1,537,122 | 1,999,999 | 844,891 | 647,435 | |
| Totals for Vaccines with bacterins/bacterial extracts/toxoids | 3,960,608 | 2,517,198 | 6,846,988 | 149,050 | 2,497,584 | 823,855 | |
| Totals for For further manufacture: bacterins & bacterial extracts | 0 | 0 | 0 | 0 | 0 | 0 | |
| Totals for For further manufacture: vaccines w bacterins/bacterial extracts/toxoids | NR | NR | 0 | 0 | 0 | 0 | |
| GRAND TOTAL | 4,337,508 | 6,291,676 | 8,384,110 | 2,149,049 | 3,342,475 | 1,471,290 | |
Appendix C:
USDA reagents used for leptospirosis potency testing.
| USDA Biologics Testing - Leptospira Reagents | ||||||||
|---|---|---|---|---|---|---|---|---|
| USDA Reagent ID Number(s) | USDA Lot ID | USDA Reagant Title(s) | Reagent Type | Animal-derived? Y/N | Species Affected | Test Used For | Notes | Expiration Date |
| CVB-DAT-0006 | IRP 653 | Leptospira canicola Challenge Culture | Challenge Culture | Yes | Hamster | SAM 609 (in vivo) | Prepared in liver tissue of hamster infected with specific Leptospira serovar | 1/26/2022 |
| BBDAT0010 | IRP 656 | Leptospira grippotyphosa Challenge Culture | Yes | SAM 617 (in vivo) | 5/11/2022 | |||
| CVB-DAT-0011 | IRP 655 | Leptospira icterohaemorrhagiae Challenge Culture | Yes | SAM 610 (in vivo) | 3/30/2022 | |||
| CVB-DAT-0012 | IRP 654 | Leptospira pomona Challenge Culture | Yes | SAM 608 (in vivo) | 2/23/2022 | |||
| CVB-DAT-0069 | IRP 523 (05) | Leptospira grippotyphosa Reference Bacterin | Reference Bacterin/Master Reference | Unknown | Unknown | SAM 626 (in vitro) | Reagents “produced by a licensee according to a confidential outline of production;” dilution adjusted based on host animal dosage (canine or porcine) | 5/31/2022 |
| CVB-DAT-0070 | IRP 542 (06) | Leptospira icterohaemorrhagiae Reference Bacterin | Unknown | SAM 627 (in vitro) | 5/31/2022 | |||
| CVB-DAT-0072 | IRP 524 (05) | Leptospira pomona Master Reference | Unknown | SAM 624 (in vitro) | 4/30/2022 | |||
| CVB-DAT-0073 | IRP 555 (07) | Leptospira canicola Master Reference | Unknown | SAM 625 (in vitro) | 4/30/2022 | |||
| CVB-DAT-0076 | IRP 04–481 | Leptospira icterohaemorrhagiae Polyclonal Antibody (LI PAb) | Polyclonal Antibody | Yes | New Zealand white rabbits | SAM 627 (in vitro) | Made by hyperimmunizing rabbits with specific Leptospira strain | 4/30/2022 |
| CVB-DAT-0077 | IRP 04–482 | Leptospira pomona Polyclonal Antibody (LP PAb) | Yes | SAM 624 (in vitro) | 4/30/2022 | |||
| CVB-DAT-0079 | IRP 491 | Leptospira canicola Polyclonal Antibody (LC PAb) | Yes | SAM 625 (in vitro) | 4/30/2022 | |||
| CVB-DAT-0098 | IRP 511–04 | Leptospira grippotyphosa Polyclonal Antibody (LG PAb) | Yes | SAM 626 (in vitro) | 4/30/2023 | |||
| BBDAT0094 | IRP 04–476 | Leptospira pomona Monoclonal Antibody (LP MAb) | Monoclonal Antibody | Yes | Mice | SAM 624 (in vitro) | Produced form hybridoma method by immunizing BALB/c mice with Leptospira strain, then collecting ascites fluid. There are two versions of the pomona strain MAb, but all methods are the same. | N/A |
| CVB-DAT-0080 | IRP 04–500 | Leptospira canicola Monoclonal Antibody (LC MAb) | Yes | SAM 625 (in vitro) | 4/30/2022 | |||
| BBDAT0101 | IRP 04–504 | Leptospira pomona Monoclonal Antibody (LP MAb) | Yes | SAM 624 (in vitro) | 4/30/2022 | |||
| CVB-DAT-0082 | IRP 04–505 | Leptospira grippotyphosa Monoclonal Antibody (LG MAb) | Yes | SAM 626 (in vitro) | 4/30/2022 | |||
| CVB-DAT-0083 | IRP 04–506 | Leptospira icterohaemorrhagiae Monoclonal Antibody (LI MAb) | Yes | SAM 627 (in vitro) | 4/30/2022 | |||
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Srinivas GB, Walker A, Rippke B. USDA regulatory guidelines and practices for veterinary Leptospira vaccine potency testing. Biologicals 2013;41:298–302. 10.1016/j.biologicals.2013.06.005. [DOI] [PubMed] [Google Scholar]
- [2].Ruby KW, Srinivas GB. Development of in vitro assays for measuring the relative potency of leptospiral bacterins containing serogroups canicola, grippotyphosa, icterohaemorrhagiae, and pomona. Biologicals 2013;41:308–14. 10.1016/j.biologicals.2013.06.007. [DOI] [PubMed] [Google Scholar]
- [3].Guerra MA. Leptospirosis: Public health perspectives. Biologicals 2013;41:295–7. 10.1016/j.biologicals.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Haake DA, Levett PN. Leptospirosis in humans. Curr Top Microbiol Immunol 2015;387:65–97. 10.1007/978-3-662-45059-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Lilenbaum W, Martins G. Leptospirosis in cattle: A challenging scenario for the understanding of the epidemiology. Transbound Emerg Dis 2014;61:63–8. 10.1111/tbed.12233. [DOI] [PubMed] [Google Scholar]
- [6].Van De Maele I, Claus A, Haesebrouck F, Daminet S. Leptospirosis in dogs: A review with emphasis on clinical aspects. Vet Rec 2008;163:409–13. 10.1136/vr.163.14.409. [DOI] [PubMed] [Google Scholar]
- [7].Code of Federal Regulations. 9 CFR Part 113 Standard Requirements, 113.101–105. n.d. https://www.ecfr.gov/current/title-9/chapter-I/subchapter-E/part-113/subject-group-ECFR275e24cb93d1fab?toc=1 (accessed January 27, 2022).
- [8].Rippke B Center for Veterinary Biologics Notice No. 15–13. 2015. [Google Scholar]
- [9].Rippke B Center for Veterinary Biologics Notice No. 17–06. 2017. [Google Scholar]
- [10].USDA. Veterinary Services Memorandum No. 800.206 2014.
- [11].USDA. SAM 624 Supplemental Assay Method for In vitro Potency Testing of Leptospira interrogans serogroup pomona Bacterins. 2017.
- [12].USDA. SAM 625 Supplemental Assay Method for In vitro Potency Testing of Leptospira interrogans serogroup canicola Bacterins 2017.
- [13].USDA. SAM 626 Supplemental Assay Method for In vitro Potency Testing of Leptospira kirschneri serogroup grippotyphosa Bacterins 2017.
- [14].USDA. SAM 627 Supplemental Assay Method for In vitro Potency Testing of Leptospira interrogans serogroup icterohaemorrhagiae Bacterins 2017.
- [15].Kulpa-Eddy J, Srinivas G, Halder M, Hill R, Brown K, Roth J, et al. Non-Animal Replacement Methods for Veterinary Vaccine Potency Testing: State of the Science and Future Directions. Procedia Vaccinol 2011;5:60–83. 10.1016/j.provac.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Stokes W, Srinivas G, McFarland R, Kulpa-Eddy J, Casey W, Walker A, et al. Report on the international workshop on alternative methods for Leptospira vaccine potency testing: State of the science and the way forward. Biologicals 2013;41:279–94. 10.1016/j.biologicals.2013.06.013. [DOI] [PubMed] [Google Scholar]
- [17].USDA Animal and Plant Health Inspection Service Annual Reports Search. n.d. https://aphis-efile.force.com/PublicSearchTool/s/annual-reports (accessed September 9, 2020).
- [18].Alt DP, Wilson-Welder J. Expansion of the in vitro assay for Leptospira potency testing to other serovars: Case study with Leptospira Hardjo. Biologicals 2013;41:323–4. 10.1016/j.biologicals.2013.06.003. [DOI] [PubMed] [Google Scholar]
- [19].Draayer HA, Bruckner L, Peña-Moctezuma A de la, Srinivas G. International regulatory requirements for Leptospira vaccine potency testing. Roundtable: Current requirements and opportunity for harmonization. Biologicals 2013;41:305–7. 10.1016/j.biologicals.2013.06.008. [DOI] [PubMed] [Google Scholar]
- [20].USDA APHIS | Reagent Data Sheets - BB (Bacteriology) n.d. https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/veterinary-biologics/biologics-regulations-and-guidance/ct_vb_dats_bact (accessed June 7, 2021).
- [21].Halder M, Barroso J, Whelan M. EURL ECVAM Recommendation on Non-Animal-Derived Antibodies. 2020. 10.2760/80554. [DOI] [Google Scholar]
- [22].Groff K, Brown J, Clippinger AJ. Modern affinity reagents: Recombinant antibodies and aptamers. Biotechnol Adv 2015;33:1787–98. 10.1016/J.BIOTECHADV.2015.10.004. [DOI] [PubMed] [Google Scholar]
- [23].Groff K, Allen D, Casey W, Clippinger A. Increasing the use of animal-free recombinant antibodies. ALTEX - Altern to Anim Exp 2020;37:309–11. 10.14573/ALTEX.2001071. [DOI] [PubMed] [Google Scholar]
- [24].Bradbury A, Plückthun A. Reproducibility: Standardize antibodies used in research. Nat 2015 5187537 2015;518:27–9. 10.1038/518027a. [DOI] [PubMed] [Google Scholar]
- [25].Innovative Science and Technology Approaches for New Drugs (ISTAND) Pilot Program | FDA n.d. https://www.fda.gov/drugs/drug-development-tool-ddt-qualification-programs/innovative-science-and-technology-approaches-new-drugs-istand-pilot-program (accessed September 17, 2021).
- [26].Medical Device Development Tools (MDDT) | FDA n.d. https://www.fda.gov/medical-devices/science-and-research-medical-devices/medical-device-development-tools-mddt (accessed August 15, 2021).


