Table 1 – Name, chemical structure and pIC50 of β-agonists.
Name and associated chemical structure of the β-agonists used in this study are given. pIC50 ± SEM, n = 3, extrapolated from radiolabeled competition binding experiments with [3H]DHA (Fig. S1) is also provided. For direct comparison, pEC50 ± SEM, n = 6 for cAMP production and β-arrestin recruitment extrapolated from curve-fitting in Fig. 1C and D is also provided.
| Agonist | Chemical structure | Binding pIC50 ± SEM (n = 3) | cAMP production EC50 ± SEM (n = 6) | β-arrestin recruitment pEC50 ± SEM (n = 6) |
|---|---|---|---|---|
| (−)-Isoproterenol |
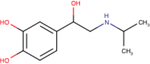
|
6.2 ± 0.1 | 7.8 ± 0.1 | 7.3 ± 0.1 |
| (−)-Epinephrine |
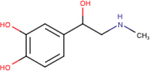
|
5.4 ± 0.2 | 6.8 ± 0.1 | 6.6 ± 0.1 |
| (−)-Norepinephrine |
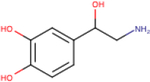
|
3.5 ± 0.3 | 5.4 ± 0.2 | 5.2 ± 0.1 |
| Fenoterol |
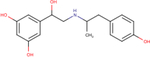
|
5.5 ± 0.2 | 6.1 ± 0.1 | 6.9 ± 0.1 |
| Ractopamine |
|
6.6 ± 0.1 | 7.8 ± 0.5 | 4.4 ± 0.6 |
| Buphenine |
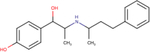
|
6.6 ± 0.1 | 8.2 ± 0.8 | 4.5 ± 0.7 |
| Ritodrine |
|
5.9 ± 0.7 | 6.1 ± 0.7 | 4.7 ± 0.5 |
| Dobutamine |
|
5.3 ± 0.1 | 6.3 ± 1.0 | nd |
| Bamethane |
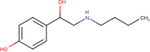
|
4.5 ± 0.1 | 5.4 ± 0.6 | nd |
| Salbutamol |
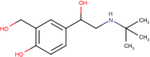
|
5.6 ± 0.1 | 6.3 ± 0.1 | 6.3 ± 0.2 |
| Salmeterol |
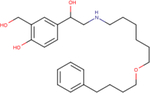
|
6.8 ± 0.0 | 8.2 ± 0.5 | 7.6 ± 0.4 |
| Higenamine |
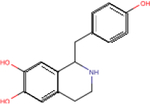
|
5.3 ± 0.1 | 5.7 ± 0.3 | 5.1 ± 0.3 |
