Abstract
Purpose
The present work was a pilot study undertaken to evaluate the effectiveness of amoxicillin and clavulanic acid impregnated plaster of paris beads for prevention of infection of third molar extraction sockets.
Materials and Methods
This was a prospective, randomized, split mouth clinical trial done on 16 patients (32 sites) who required surgical extraction of mandibular third molars. Control arm patients were given Tab. amoxicillin 500 mg with clavulanic acid 125 mg (Tab. Klavimed 625 mg, Indomed, India), thrice daily for 3 days after extraction, whereas test arm patients received Antibiotic Impregnated Microbeads (AIM), containing Amoxicillin 500 mg and Clavulanic Acid 100 mg placed in situ in the extraction socket. The primary outcome parameter was infection and the secondary outcome parameters were pain, trismus, swelling and wound healing.
Results
None of the patients in either group had post operative infection. There was no significant difference in pain intensity between the two groups (1st day p = 0.41; 3rd day p = 0.38, 7th day p = 0.37). Both the groups were also similar with respect to swelling (p = 0.596, 0.146, 0.871, 0.820 on 1st, 3rd, 7th, 15th post-op day ,respectively).
Conclusion
Amoxycillin with clavulanic acid impregnated PoP beads appears to be as effective as oral 3 day amoxicillin with clavulanic acid regime for prevention of 3 M socket infection.
Keywords: Antibiotic impregnated PoP beads, Local drug delivery, 3 M socket infection
Introduction
Third molar (3 M) surgeries are the most common surgical procedures performed by Oral and Maxillofacial surgeons. Common post operative complications associated with 3 M surgeries are pain, trismus, swelling and infection. Antibiotics are commonly administered, whether oral or parenteral to reduce the chances of post-op infection of the extraction site. Penicillin’s are the most frequently used antibiotics for preventing socket infection. Both oral and parenteral Penicillins are associated with significant number of problems like patient compliance, gastrointestinal disturbance and pain of injection. To overcome these complications, authors have reported single-dose prophylactic antibiotic administration for 3 M surgeries [1, 2].
Local drug delivery at the site of surgery has the potential of having advantages of antibiotic without the major shortcomings of oral/parenteral administration. The zero-order release kinetics of antibiotics from local delivery systems may be very attractive to prevent development of antibiotic resistance due to the absence of long-term, low concentration tail-release [3]. Local drug delivery of antibiotics has been tried primarily in orthopedic surgery with poly methyl methacrylate (PMMA), plaster of paris (PoP), resorbable sponge and polylactates for the management of osteomyelitis with favorable results [4]. Resorbable medium like PoP appears to have advantages like single-stage surgery, maximum elution within 24–48 h and biocompatibility. In vivo use and in vitro elution characteristics have been studied with penicillins, gentamicin, vancomycin, teicoplanin, clindamycin, amikacin and ceftiofur [5–10].
To the best of our knowledge, local drug delivery of amoxicillin utilizing PoP has not been tried after 3 M surgery till now. The present study was undertaken as a pilot study to evaluate the clinical effectiveness of amoxicillin and clavulanic acid impregnated PoP beads in controlling post-operative socket infection after 3 M surgery. The study began with a null hypothesis that there is no difference between locally delivered amoxicillin with clavulanic acid and orally administered 3 days amoxicillin with clavulanic acid regime for prevention of post-operative infection of 3 M extraction site.
Materials and Methods
This study was carried out as a randomized controlled clinical trial: split mouth design (Fig. 1). The estimated sample size was calculated by taking the means of mouth opening, facial swelling and wound dehiscence from a previous similar study conducted by Jiminez et al. [11] with a confidence level of 95% and power of 80%. The calculated sample size was 13 patients (26 operating sites). 20% was added to the sample size from the start of the study to compensate for drop-out. Therefore, 16 patients (32 operating sites) who required removal of bilateral impacted lower third molars were recruited from the outpatient department of oral and maxillofacial surgery after fulfilling the inclusion and exclusion criteria. The inclusion criteria were patients with age ranging from 18 to 60 years having bilateral mandibular impacted third molars with no systemic disorders and who have second molar present in the oral cavity. The exclusion criteria were heavy smokers, uncontrolled systemic conditions, pathologies, and infection at the site of surgery, missing second molar tooth and patients not ready to give the written consent. The selected patients were randomized and allocated to one of the groups. Randomization was done by envelope method; 16 sets of unmarked envelopes were prepared. Each set contained two envelopes, one having a paper slip with ‘Study’ written on it and the other having ‘Control’ written on it. The side to be operated first was decided after discussion with the patient. The nurse opened one of the envelopes and conveyed whether the side would be study side or control side. All operations were performed by qualified maxillofacial surgeon under local anesthesia consisting of 2% lidocaine hydrochloride with 1:80,000 (Lignox, Warrant Pharmaceutical, India). A modified Ward's incision was used in all the cases. The impacted tooth was removed by standard technique. The flap was repositioned and sutured with 4–0 silk suture (Truesilk, Suture India, India). Both the groups received the same postoperative prescription except the antibiotic. In group A (control group), the patients were given Tab. Amoxicillin 500 mg with clavulanic acid 125 mg (Tab. Klavimed 625 mg, Indomed, India), thrice daily for 3 days. Group B (Study Group) patients received Antibiotic Impregnated Microbeads (AIM), containing amoxicillin 500 mg and clavulanic acid 100 mg placed in situ (Fig. 2) in the extraction socket before primary closure (Fig. 3).
Fig. 1.
Study design flow-chart
Fig. 2.

Antibiotic Impregnated Microbeads (AIM) placed in the extraction socket
Fig. 3.
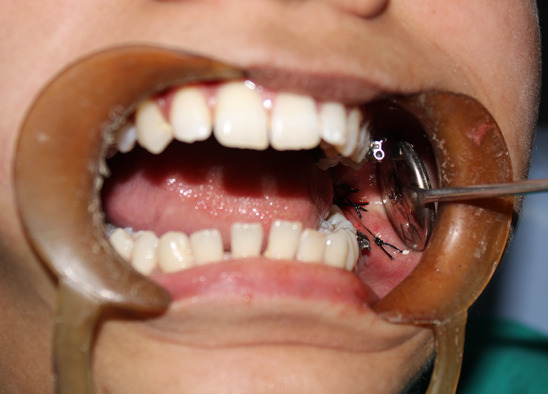
Wound closure after AIM placement
Determining the Amount of Medical Grade Calcium Sulfate Hemihydrate (MGCSH) to Fabricate AIM
The amount was predetermined by mounting five different average sized extracted third molars in alginate. While mounting one of the cusps was not embedded in the alginate. The tooth was removed from the alginate and the cavity thus created was filled with medical grade calcium sulfate hemihydrate to determine the amount of the powder required for filling the cavity; the powder was removed and weighed.
Mean weight of MGCSH: 1.5 gm ± 0.2 gm.
Preparation of AIM
AIM was prepared by mixing the MGCSH with antibiotic powder sourced from inj. Blumox-CA (500 mg of amoxicillin potassium and 100 mg clavulanic acid) and sterile water in a 3:2 powder to liquid ratio (Fig. 4, Fig. 5). The two were mixed thoroughly to form a pliable paste which was poured in a tray (Fig. 6) containing voids of size 3 × 1 mm (fabricated by addition silicon impression material) to form microbeads of 3 x 1 mm (Fig. 7, Fig. 8). The MGCSH was autoclaved prior to use and the mixing and pouring were done in sterile condition.
Fig. 4.

Mixture of MGCSH and antibiotic powder
Fig. 5.

Paste formed after mixing powder with liquid
Fig. 6.

Silicon mold
Fig. 7.
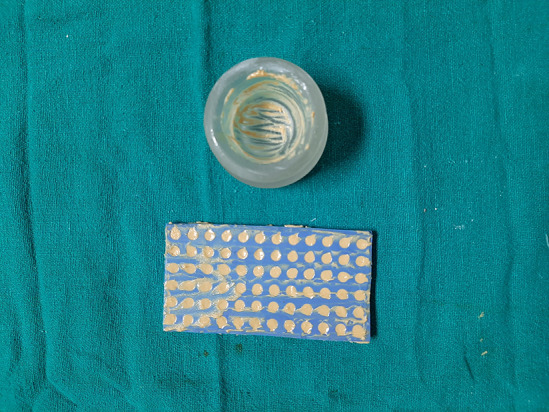
Paste loaded into the silicon mold
Fig. 8.

Beads collected after drying
All work was conducted in accordance with the Declaration of Helsinki (1964). All patients were informed about the whole procedure and detailed written informed consent was taken. Ethics clearance was taken from the institutional ethics review board (SDC&RI/IEC/20017/016).
Outcome Parameters
The primary outcome parameter was infection on postoperative evaluation on 1st, 3rd, 7th and 15th day. Infection was defined as the concurrent presence of at least three of the following parameters (pain at the extraction site, trismus, localized swelling and suppuration) with the presence of suppuration being mandatory.
Secondary outcome parameter assessed were Pain, Trismus, Swelling and Wound Healing
The patients were asked to rate their pain on a printed 10-cm VAS where a score of 0 indicated “no pain” and 10, “intolerable pain”. Trismus was evaluated by measuring the interincisal distance between the incisal edge of the upper and lower central incisors using a caliper at maximum mouth opening (cm). Swelling was assessed by calculating facial size. The length from the corner of the mouth to the attachment of the ear lobe (anterio-posteriorly) and corner of the eye to the angle of the mandible (superio-inferiorly) was measured and added to derive facial size. Facial swelling was calculated according to the following formula
Wound healing was assessed based on the criteria given by Landry et al. (Table 1) [12].
Table 1.
Healing criteria by Landry et al. [10]
| Healing index | Tissue color | Bleeding on palpation | Granulation tissue | Incision margin | Suppuration |
|---|---|---|---|---|---|
| 1—Very Poor: 2 or more signs are present | ≥ 50% of red gingiva | Yes | Yes | Not epithelialized, with loss of epithelium beyond incision margin | Yes |
| 2—Poor | ≥ 50% of red gingiva | Yes | Yes | Not epithelialized, with exposed connective tissue | No |
| 3—Good | 25—50% of red gingiva | No | No | No exposed connective tissue | No |
| 4—Very Good | < 25% of red gingiva | No | No | No exposed connective tissue | No |
| 5—Excellent | all pink tissues | No | No | No exposed connective tissue | No |
Exploratory data analysis techniques including the mean, median and standard deviation were used for the evaluation of the results of this study. The comparison of difficulty index between the groups was analyzed using Chi-square test. For the assessment of pain on day 1, Fisher exact test was used and for day 3 and 7, chi-square test was used. For the assessment of swelling and inter incisal distance student t test was used.
Results
Out of 16 patients three patient were dropped from the study as they were unable to come for the follow-up. The distribution of demographic variables and confounding variables between the groups was analyzed using Chi-square test and student t test. The sample consisted of 13 patients, and the mean age was 27 ± 7.5 years with seven (53.8%) females and six (46.2%) males (Table 2). The difficulty index was assessed based on modified Pederson index. Control and study groups were statistically similar in terms of distribution of slightly difficult and very difficult cases, however, the control group had significantly more moderately difficult cases (p = 0.36) (Table 2).
Table 2.
Demographic data and difficulty index
| Parameters | Mean | Std deviation |
|---|---|---|
| Age | 27 | 7.5 |
| Gender | N | % |
| Male | 6 | 46.2 |
| Female | 7 | 53.8 |
| Difficulty index | Group A | Group B | p value |
|---|---|---|---|
| Slight difficult | 3 | 9 | 0.036 Insignificant |
| Moderate difficult | 8 | 2 | |
| Very difficult | 2 | 2 |
Infection, the primary outcome variable, was absent in both the groups throughout the assessment period (Table 3).
Table 3.
Outcome variables after lower third molar extraction
| Days | Day 1 | Day 3 | Day 7 | Day 15 |
|---|---|---|---|---|
| Primary outcome | ||||
| Infection | ||||
| Group A (Control Group) | Absent | Absent | Absent | Absent |
| Group B (Study Group) | Absent | Absent | Absent | Absent |
| Secondary outcome | ||||||||
|---|---|---|---|---|---|---|---|---|
| Score | Group A | Group B | Group A | Group B | Group A | Group B | Group A | Group B |
| Pain# | ||||||||
| 0 | 6(46.0%) | 8(61.5%) | 9(69.2%) | 10(76.9%) | 11(84.6%) | 12(92.3%) | 13(100%) | 13(100%) |
| 1 | 2(15.4%) | 1(07.7%) | 2(15.4%) | 2(15.4%) | 2(15.4%) | 1(07.7%) | – | – |
| 2 | 5(38.6%) | 2(15.4%) | 2(15.4%) | 1(07.7%) | – | – | – | – |
| 3 | – | 1(07.7%) | – | – | – | – | – | – |
| 4 | – | 1(07.7%) | – | – | – | – | – | – |
| Group A | Group B | Group A | Group B | Group A | Group B | Group A | Group B | |
|---|---|---|---|---|---|---|---|---|
| Swelling* | ||||||||
| Mean value in mm | 21.685 | 21.462 | 22.646 | 21.615 | 21.146 | 21.231 | 21.108 | 21.231 |
| Std. Dev | 1.2151 | 0.8771 | 1.9221 | 1.5566 | 1.3233 | 1.3009 | 1.4256 | 1.3009 |
| t value | 0.537 | 1.503 | – 0.164 | –0.230 | ||||
| p value | 0.596 | 0.146 | 0.871 | 0.820 | ||||
| Group A | Group B | Group A | Group B | Group A | Group B | Group A | Group B | |
|---|---|---|---|---|---|---|---|---|
| Inter incisal distance* | ||||||||
| Mean value in mm | 43.23 | 43.46 | 32.31 | 32.00 | 35.46 | 35.38 | 42.62 | 42.38 |
| Std. dev | 5.449 | 5.254 | 6.156 | 6.364 | 5.768 | 5.752 | 5.620 | 5.881 |
| t value | – 0.110 | 0.125 | 0.034 | 0.102 | ||||
| p value | 0.913 | 0.901 | 0.973 | 0.919 | ||||
*Significant if p ≤ 0.05
#Day1: Fisher exact test value—3.905; p = 0.419
Day 3: Chi-square statistic is 0.386, p = 0 .824496
Day 7: Chi-square statistic is 0.3768, p = 0 .539315
The secondary outcome variables were pain, swelling, trismus and wound healing. On 1st post-op day, in control group, 46.2% patients had score 0, whereas in study group, 61.5% had score 0. As time progressed, the proportion of patients with score 0 increased with study side having higher percentage at each time interval. By 15th day all patients had score 0 in both the groups. There was no significant difference in pain intensity between the two groups (1st day p = 0.41; 3rd day p = 0.38, 7th day p = 0.37) (Table 3). In both the groups, increase in facial size was observed after surgery (Table 3). However, the difference between the groups was statistically insignificant (p = 0.596, 0.146, 0.871, 0.820 on days 1st, 3rd, 7th, 15th, respectively).
A significant decrease in mouth opening (Table 3) was observed in patients from both treatment groups. The highest decrease in interincisal distance was recorded on third postoperative day and returned to presurgical interincisal distance on 15th day, the difference between the groups was not statistically significant. Both the groups had similar wound healing. On the 1st and 3rd day the healing index was 3, whereas on 7th day, healing index 5 was observed in both the groups.
Discussion
Infection of the 3 M extraction site is a chief concern for oral and maxillofacial surgeons. The overall incidence of infection ranges between 10 and 25%. A reduction in the rate of infection by 70% has been reported with the use of antibiotics when compared to the rate of infection without antibiotics. Though there is still no universal consensus whether antibiotics should be administered or not after 3 M surgery, the general leaning is toward administering antibiotics.
Pre operative single-dose regime antibiotic has been studied by authors for third molar surgeries with promising results [1, 2]. This regime is based on the premise that it is best to have the antibiotic present in circulation at the time of formation of blood clot [11].
The present study was a randomized split mouth trial undertaken to evaluate the clinical effectiveness of amoxicillin and clavulanic acid impregnated PoP beads in preventing infection of the 3 M extraction sockets.
PoP was chosen for local drug delivery because it is simple to use, cheap, stable and does not require a second surgery for removal of beads [14]. It is also easily sterilized, well tolerated by tissues and does not incite inflammatory reaction [15]. PoP has been used as an osteoconductive graft material for socket preservation proving its acceptability in oral environment [16, 17]. It has also been used in orthopedic surgery as a vehicle for local delivery of antibiotics for the management of infections of bone [18]. PoP appears to have favorable antibiotic elution characteristics for use after 3 M surgery. Long-term presence of antibiotics is not necessary, and the most critical time for exposing the wound to antibiotics is during and immediately after 3 M surgery [19]. Comparing favorably to this is the rapid release of antibiotics from PoP in contrast to polymethylmethacrylate which elutes antibiotics for longer duration [10]. Most of the studies report a bolus release of antibiotics within 24 h by PoP pellets [15, 20, 21]. An important parameter for local drug delivery is the stability of the drug at body temperature. At 37 degrees Celsius, sodium amoxicillin has been shown to be active for 3 days compared to amoxicillin trihydrate which is active for 7 days [15]. Elution of amoxicillin for first 48 h has been reported to maintain levels more than the minimum inhibitory concentration (MIC) required for susceptible pathogens [10]. The same study also reported maximum elution of amoxicillin plus clavulanic acid during the first 3 h reducing to no activity after 48 h and the reason suggested was that it could be due to limited release of clavulanic acid or its inactivation with time within the pellet. This release profile of amoxicillin and amoxicillin with clavulanic acid may be sufficient for 3 M surgeries as single-dose prophylactic antibiotic regime for 3 M surgeries has been shown to be effective in preventing post operative infection [19, 22].
The incorporation of the PoP beads in the socket wound was simple. The pellets were radio-opaque which allows for radiographic monitoring of resorption, though this was not one of the parameters in the present study. PoP was used as local antibiotic delivery vehicle in 13 patients. None of the patients had post operative socket infection. The control group also did not have any case of post-op infection. There were no other similar studies evaluating the effectiveness of antibiotic impregnated PoP for prevention of post-op socket infection. However, many studies have reported low rate of infection with the use of antibiotics ranging from 3 to 5% [23]. A meta-analysis for Cochrane by Lodi G et al. [24] reported that antibiotics after 3 M extractions reduce the risk of infection by approximately 70%.
The distribution of demographic variables between the two groups was similar. However, there was a predominance of ‘slightly difficult’ cases in the study group and a predominance of ‘moderately difficult’ cases in control group. This may have had a confounding effect on the present study.
The statistical difference between the groups with regard to pain, trismus and swelling was not significant. The highest pain score in both the groups on day 7 was one, with one patient in study group and two patients in control group with that score. The highest score on day 3 was 2 in both the groups. The low incidence of pain compares favorably to studies by [25–28].
Most studies indicate that maximum swelling is reached in 2–3 days and complete regression happens in a weeks’ time [29]. Both the groups of the present study also demonstrated a similar trend. Trismus was maximum by third day in both the groups and returned to preoperative state by 15th day. This compares favorably to the existing literature [29]. Both the groups had similar and satisfactory wound healing scores by 7th day. Studies utilizing PoP for socket preservation have reported no deleterious effects on healing [16, 17].
A major drawback of the present study is the small sample size. The present study only serves the purpose of a pilot study. Another drawback is that eluted antibiotic in the socket was not measured. Elution characteristics may be affected by size of pellet, storage of pellets and local tissue environment. Clinically, however, the test group appears to have performed, as well as the control group. Validation of the results over a larger sample size is required.
Conclusion
Results of the present study suggest that antibiotic impregnated PoP beads are biocompatible, easy to prepare and use and may be an effective alternative to oral and parenteral antibiotic regimes for 3 M surgeries. Local drug delivery of antibiotics after third molar extraction may offer greater benefits than systemic antibiotic administration.
Funding
The authors have not received any funding from any sources.
Data availability
Master data available.
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Ethical approval
Approved by iIECSDCRI. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Consent to participate
Written consent was taken from all the participants.
Consent for publication
Publication does not include any patient photographs.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Dinesh Kumar Verma, Email: dineshverma@yahoo.com.
Shallu Bansal, Email: drshallu23@yahoo.com.
Kaushal Charan Pahari, Email: kaushalcpahari@gmail.com.
References
- 1.López-Cedrún JL, Pijoan JI, Fernández S, Santamaria J, Hernandez G. Efficacy of amoxicillin treatment in preventing postoperative complications in patients undergoing third molar surgery: a prospective, randomized, double-blind controlled study. J Oral Maxillofac Surg. 2011;69:e5–14. doi: 10.1016/j.joms.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 2.Monaco G, Tavernese L, Agostini R, Marchetti C. Evaluation of antibiotic prophylaxis in reducing postoperative infection after mandibular third molar extraction in young patients. J Oral Maxillofac Surg. 2009;67:1467–1472. doi: 10.1016/j.joms.2008.12.066. [DOI] [PubMed] [Google Scholar]
- 3.Kluin OS, Van Der Mei HC, Busscher HJ, Neut D. Biodegradable vs non-biodegradable antibiotic delivery devices in the treatment of osteomyelitis. Expert Opin Drug Deliv. 2013;10:341–351. doi: 10.1517/17425247.2013.751371. [DOI] [PubMed] [Google Scholar]
- 4.McConoughey SJ, Howlin RP, Wiseman J, Stoodley P, Calhoun JH. Comparing PMMA and calcium sulfate as carriers for the local delivery of antibiotics to infected surgical sites. J Biomed Mater Res - Part B Appl Biomater. 2015;103:870–877. doi: 10.1002/jbm.b.33247. [DOI] [PubMed] [Google Scholar]
- 5.Ethell MT, Bennett RA, Brown MP, Merritt K, Davidson JS, Tran T. In vitro elution of gentamicin, amikacin, and ceftiofur from polymethylmethacrylate and hydroxyapatite cement. Vet Surg. 2000;29:375–382. doi: 10.1053/jvet.2000.7535. [DOI] [PubMed] [Google Scholar]
- 6.Wichelhaus TA. Elution characteristics of vancomycin, teicoplanin, gentamicin and clindamycin from calcium sulphate beads. J Antimicrob Chemother. 2001;48:117–119. doi: 10.1093/jac/48.1.117. [DOI] [PubMed] [Google Scholar]
- 7.Liu SJ, Ueng SW, Chan EC, Lin SS, Tsai CH, Wei FC, Shih CH. In vitro elution of vancomycin from biodegradable beads. J Biomed Mater Res. 1999;48:613–620. doi: 10.1002/(SICI)1097-4636(1999)48:5<613::AID-JBM4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 8.Atilla A, Boothe HW, Tollett M, Duran S, Diaz DC, Sofge J, Boothe DM. In vitro elution of amikacin and vancomycin from impregnated plaster of Paris beads. Vet Surg. 2010;39:715–721. doi: 10.1111/j.1532-950X.2009.00632.x. [DOI] [PubMed] [Google Scholar]
- 9.Parker AC, Smith JK, Courtney HS, Haggard WO. Evaluation of two sources of calcium sulfate for a local drug delivery system: a pilot study. Clin Orthop Relat Res. 2011;469:3008–3015. doi: 10.1007/s11999-011-1911-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bowyer GW, Cumberland N. Antibiotic release from impregnated pellets and beads. J Trauma. 1994;36:331–335. doi: 10.1097/00005373-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 11.Madrazo-Jiménez M, Rodríguez-Caballero Á, Serrera-Figallo MÁ, Garrido-Serrano R, Gutiérrez-Corrales A, Gutiérrez-Pérez JL, Torres-Lagares D. The effects of a topical gel containing chitosan, 0,2% chlorhexidine, allantoin and despanthenol on the wound healing process subsequent to impacted lower third molar extraction. Med Oral Patol Oral Cir Bucal. 2016;21:e696–e702. doi: 10.4317/medoral.21281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Landry RG, Turnbull RS, Howley T. Effectiveness of benzydamyne HCl in the treatment of periodontal post-surgical patients. Res Clin Forums. 1988;10:105–118. [Google Scholar]
- 13.Woods RK, Dellinger EP. Current guidelines for antibiotic prophylaxis of surgical wounds. Am Fam Physician. 1998;57:2731–2740. [PubMed] [Google Scholar]
- 14.Peltier LF. The use of plaster of Paris to fill large defects in bone: a preliminary report. 1959. Clin Orthop Relat Res. 2001;382:3–5. doi: 10.1097/00003086-200101000-00002. [DOI] [PubMed] [Google Scholar]
- 15.Mousset B, Benoit MA, Delloye C, Bouillet R, Gillard J. Biodegradable implants for potential use in bone infection. an in vitro study of antibiotic-loaded calcium sulphate. Int Orthop. 1995;19:157–61. doi: 10.1007/BF00181861. [DOI] [PubMed] [Google Scholar]
- 16.Machtei EE, Mayer Y, Horwitz J, Zigdon-Giladi H. Prospective randomized controlled clinical trial to compare hard tissue changes following socket preservation using alloplasts, xenografts vs no grafting: clinical and histological findings. Clin Implant Dent Relat Res. 2019;21:14–20. doi: 10.1111/cid.12707. [DOI] [PubMed] [Google Scholar]
- 17.Yahav A, Kurtzman GM, Katzap M, Dudek D, Baranes D. Bone regeneration: properties and clinical applications of biphasic calcium sulfate. Dent Clin North Am. 2020;64:453–472. doi: 10.1016/j.cden.2019.12.006. [DOI] [PubMed] [Google Scholar]
- 18.Maale GE, Eager JJ, Mohammadi DK, Calderon FA. Elution profiles of synthetic CaSO4 hemihydrate beads loaded with vancomycin and tobramycin. Eur J Drug Metab Pharmacokinet. 2020 doi: 10.1007/s13318-020-00622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cervino G, Cicciù M, Biondi A, Bocchieri S, Herford AS, Laino L, Fiorillo L. Antibiotic prophylaxis on third molar extraction: systematic review of recent data. Antibiotics. 2019;8:1–14. doi: 10.3390/antibiotics8020053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Peltier L. The use of plaster of Paris to fill large defects in bone. Am J Surg. 1959;97:11–15. doi: 10.1016/0002-9610(59)90305-8. [DOI] [PubMed] [Google Scholar]
- 21.Van Hal SJ, Paterson DL, Gosbell IB. Emergence of daptomycin resistance following vancomycin-unresponsive Staphylococcus aureus bacteraemia in a daptomycin-naive patient—a review of the literature. Eur J Clin Microbiol Infect Dis. 2011;30:603–610. doi: 10.1007/s10096-010-1128-3. [DOI] [PubMed] [Google Scholar]
- 22.Bezerra TP, Studart-Soares EC, Scaparo HC, Pita-Neto IC, Batista SHB, Fonteles CSR. Prophylaxis versus placebo treatment for infective and inflammatory complications of surgical third molar removal: a split-mouth, double-blind, controlled, clinical trial with amoxicillin (500 mg) J Oral Maxillofac Surg. 2011;69:e333–e339. doi: 10.1016/j.joms.2011.03.055. [DOI] [PubMed] [Google Scholar]
- 23.Susarla SM, Blaeser BF, Magalnick D. Third molar surgery and associated complications. Oral Maxillofac Surg Clin North Am. 2003;15:177–186. doi: 10.1016/S1042-3699(02)00102-4. [DOI] [PubMed] [Google Scholar]
- 24.Lodi G, Figini L, Sardella A, Carrassi A, Del Fabbro M, Furness S. Antibiotics to prevent complications following tooth extractions. Cochrane Database Syst Rev. 2012;11:CD3811. doi: 10.1002/14651858.CD003811.pub2. [DOI] [PubMed] [Google Scholar]
- 25.Arteagoitia I, Diez A, Barbier L, Santamaría G, Santamaría J. Efficacy of amoxicillin/clavulanic acid in preventing infectious and inflammatory complications following impacted mandibular third molar extraction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:e11–e18. doi: 10.1016/j.tripleo.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 26.Luaces-Rey R, Arenaz-Bua J, Lopez-Cedrun- Cembranos JL, Martinez-Roca C, Pertega-Diaz S, Sironvalle-Soliva S. Efficacy and safety comparison of two amoxicillin administration schedules after third molar removal. a randomized, double-blind and controlled clinical trial. Med Oral Patol Oral Cir Bucal. 2010;15:633. doi: 10.4317/medoral.15.e633. [DOI] [PubMed] [Google Scholar]
- 27.Bello SA, Adeyemo WL, Bamgbose BO, Obi EV, Adeyinka AA. Effect of age, impaction types and operative time on inflammatory tissue reactions following lower third molar surgery. Head Face Med. 2011;7:1–8. doi: 10.1186/1746-160X-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patil C, Jadhav AKR, Bhola N, Borle RM, Mishra A. Piezo surgery vs bur in impacted mandibular third molar surgery: evaluation of postoperative sequelae. J Oral Biol Craniofacial Res. 2019;9:259–62. doi: 10.1016/j.jobcr.2019.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arakji H, Shokry M, Aboelsaad N. Comparison of piezosurgery and conventional rotary instruments for removal of impacted mandibular third molars: a randomized controlled clinical and radiographic trial. Int J Dent. 2016;2016:8169356. doi: 10.1155/2016/8169356. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Master data available.



