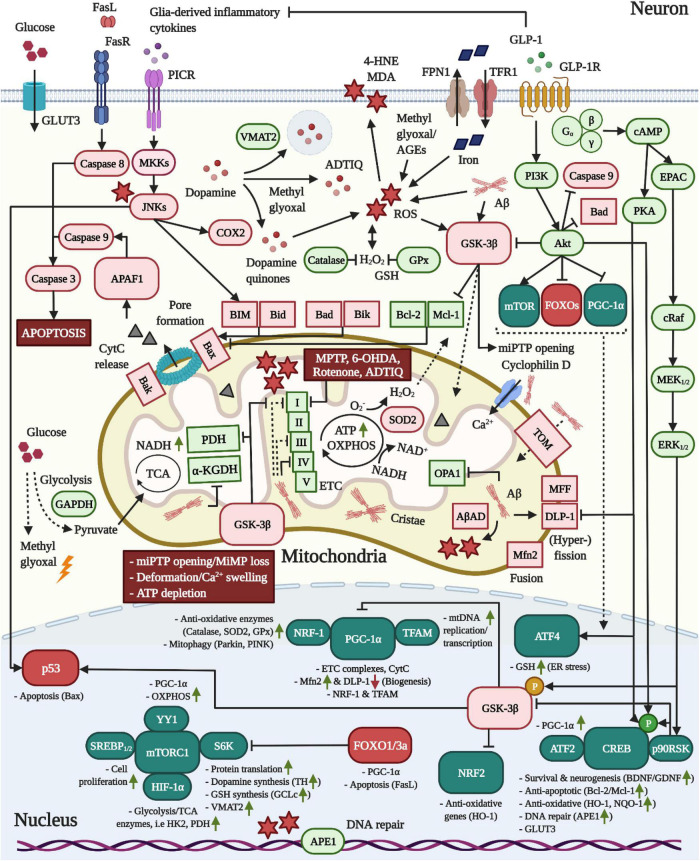
FIGURE 2.
Pro-mitochondrial, anti-oxidative and anti-apoptotic effects of GLP-1 in neurons. 1 In AD, Aβ is translocated into mitochondria via TOM and accumulates in cristae, leading to elevated ROS production through the interaction with AβAD and the impairment of the TCA enzymes PDH/α-KGDH as well as complex VI, but also I and II, of the ETC. Moreover, Aβ triggers mitochondrial Ca2+ instream and swelling by binding to cyclophilin D and stimulates mitochondrial fragmentation by altering the expression of fusion/fission-modulating proteins. 2 As common for both neurodegenerative diseases, pro-inflammatory cytokine signaling across PICRs stimulates JNK to activate BIM and Bax-expression via p53. While dopamine is packaged into synaptic vesicles by VMAT2 in dopaminergic neurons, JNK may induce COX2 to encourage the production of reactive dopamine quinones. Pathologic alterations in the expression and localization of GAPDH as well as insulin resistance-associated impairments in the expression of glycolytic enzymes may accelerate the build-up of the AGE and ROS-generating compound methyl glyoxal. The latter was shown to react with dopamine to create ADTIQ, which amasses in nigrostriatal brain areas and, similar to the PD-toxins MPTP, 6-OHDA or rotenone, inhibits complex I of the ETC to stimulate ROS production in neurons. Metal ion accumulation in the brain, in particular the iron-mediated ROS production, lipid peroxidation, mitochondrial dysfunction and ferroptosis are implicated in AD and PD. 3 Crucially, as apparent in AD, Aβ and ROS activate GSK-3β, which promotes the trafficking of GSK-3β into mitochondria to induce the opening of miPTPs, interfere with ATP production/OXPHOS by inhibiting PDH and ETC complexes and drive apoptosis by stimulating the p53-mediated synthesis of Bax and inactivating the anti-apoptotic Mcl-1. GSK-3β further suppresses NRF2-driven anti-oxidative gene transcription and elicits the degradation of PGC-1α via SCF-Cdc4 E3 ligase. 4 Metabolic stress following TCA/OXPHOS/ETC impairments and enhanced ROS load ultimately trigger miPTP opening/Ca2+ deregulation, deformation, MMP loss, ATP depletion and Bax/Bak-mediated pore formation in mitochondria, resulting in APAF1/Caspase 9/Caspase 3-mediated apoptosis. 5 The induction of the GLP-1R prevents all of the pathological alterations in neurons described above. First, the activation of the survival modulator Akt leads to the direct inactivation of GSK-3β, caspase 3, Bad and FOXOs. The Akt-induced stimulation of mTOR/mTORC1, in conjunction with various other transcription factors, augments the global protein translation, including that of the dopamine-synthesizing TH and VMAT2 in dopaminergic neurons, the GSH-producing GCLc, the mitochondrial biogenesis and fusion/fission-navigating PGC-1α as well as glycolytic/TCA enzyme expression. Notably, Akt further phosphorylates HKII to recruit it to the outer mitochondrial membrane to prevent miPTP opening, whereas GSK-3β induces the liberation of HKII, evoking the opposite result (not shown) (Rasola et al., 2010). Second, cAMP/PKA-signaling inhibits DLP-1, thus suppressing mitochondrial fragmentation. Third, PI3K/Akt, cAMP/PKA, and MEK/ERK-signaling lead to the induction of CREB to improve BDNF/GDNF expression (chapter “Other growth factors”), elevate the expression of anti-apoptotic Bcl-2/Mcl-1, upregulate anti-oxidative defense genes, and encourage deoxyribonucleic acid (DNA) repair via APE1. Fourth, GLP-1 blocks pro-inflammatory cytokine production by glial cells (chapter “Inflammation”) and, hence, PICR/JNK-signaling in neurons. Given the pro-mitochondrial and dopamine-enhancing effects, animal and clinical studies support the benefits of GLP-1 treatment in PD (see chapter “GLP-1 mimetics rescue nigrostriatal dopamine neuron death and dopamine depletion in PD”). For the anti-ferroptosis-associated effects of GLP-1 in AD and PD, see section GLP-1 analogs protect from iron and dopamine-induced oxidative stress and ferroptosis.