Figure 4.
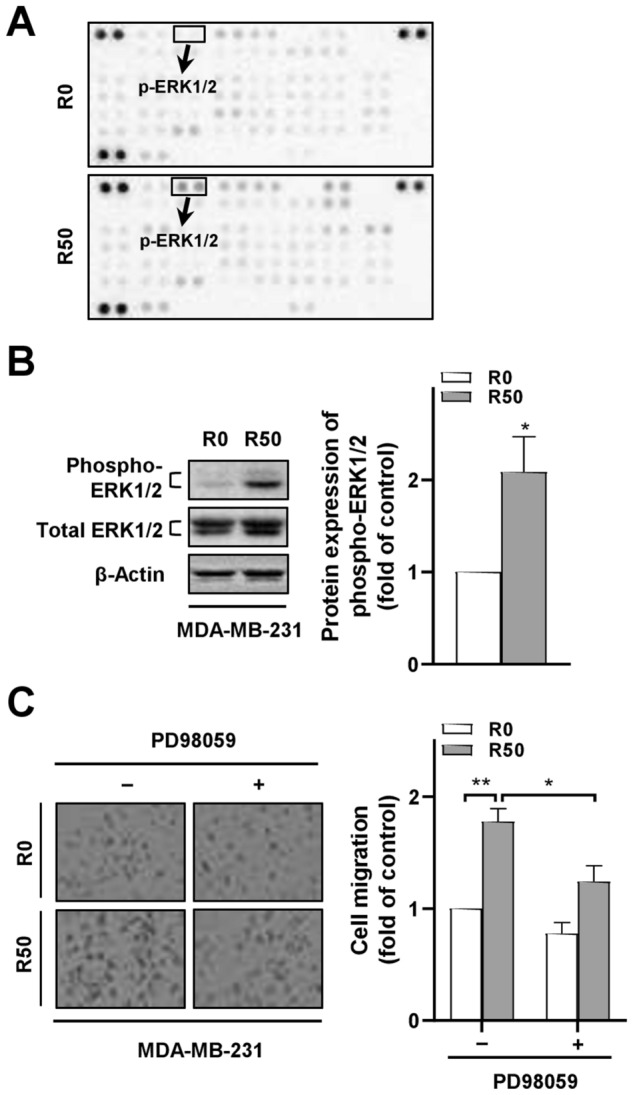
ERK phosphorylation in breast cancer cells after co-culture with resistin-stimulated ADSCs mediated the cancer cell migration ability. The isolated ADSCs were treated with resistin at 0 and 50 ng/ml (denoted as R0 and R50, respectively) for 48 h, followed by co-culture with breast cancer cells in the transwell model for another 72 h before the following analyses. (A) Total protein lysates from MDA-MB-231 cells after co-culture with R-ADSCs (R50) or control ADSCs (R0) were collected and analyzed by phospho-kinase proteome array. A total of 43 phosphorylation sites were detected with duplicates for each protein. The position of phospho-ERK1/2 (p-ERK1/2) on the array was highlighted. (B) MDA-MB-231 cells after co-culture with R-ADSCs (R50) or control ADSCs (R0) were collected and analyzed for the protein expression of phospho-ERK1/2 and total ERK1/2 by Western blot. The original blot images in (A) and (B) were available in Supplementary Information. (C) PD98059 (5 μM) was added (+) or omitted (–) during the co-culture of MDA-MB-231 with R-ADSCs (R50) or control ADSCs (R0). After the co-culture, MDA-MB-231 cells were collected and evaluated by cell migration assay. Data were obtained from three independent experiments and presented as mean ± SEM. Statistical difference was determined by t-test comparing R50 group versus R0 group as control, or comparing R50 group with PD98059 ( +) versus R50 group without PD98059 (–). *p < 0.05; **p < 0.01.
