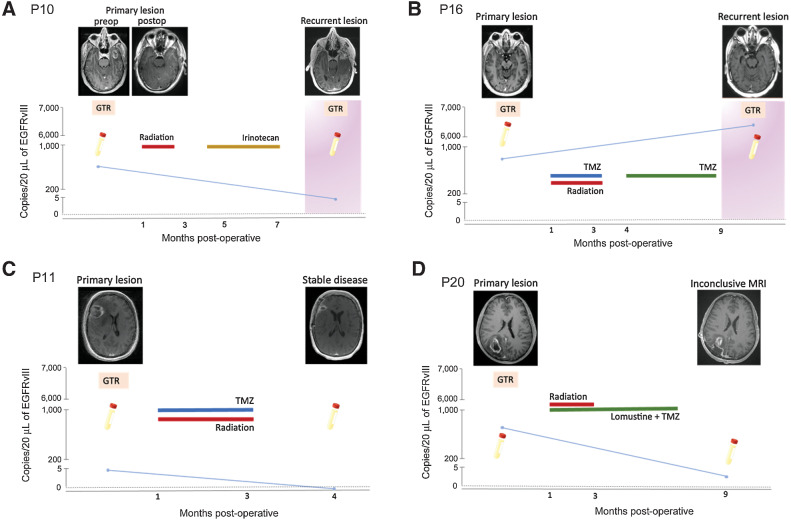
Figure 5.
Detection of EGFRvIII mutation using optimized plasma-based assay in patients with different clinical outcomes. A–D,EGFRvIII mutation (copies/20 μL) in serial plasma samples obtained from four glioma patients are plotted against time (months postoperatively). Patient cases represent different clinical outcomes: recurrent disease (A and B), stable disease (C), and inconclusive MRI (D), that is, inability to clearly delineate pseudo-progression from true tumor progression. T1-weighted, contrast-enhanced MRI images are provided for different clinical timepoints. Surgical procedures are indicated using an orange square (GTR = gross total resection). Disease progression (tumor recurrence) is indicated using a pink background. Treatment courses for each patient are outlined.