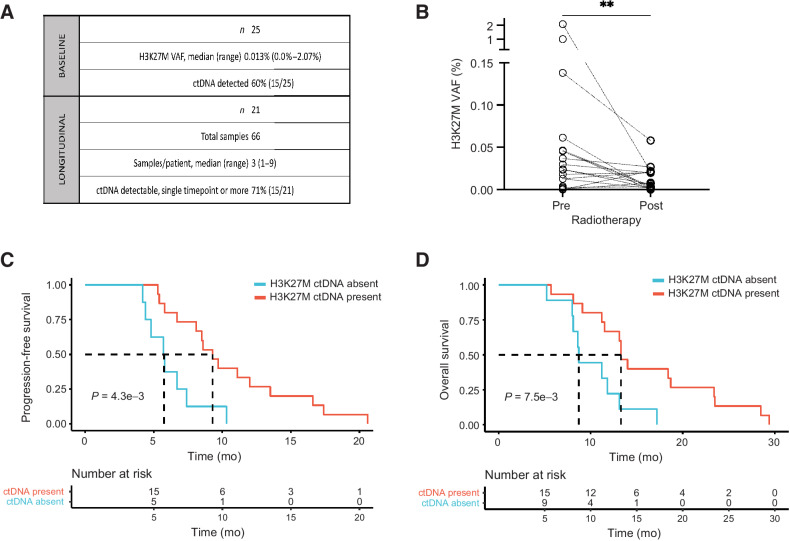
Figure 5.
H3K27M-mutant plasma ctDNA associates with clinical outcomes in DIPG. A, Summary table with baseline and longitudinal plasma ctDNA collection in PNOC003. B, Change in plasma H3K27M-mutant ctDNA VAF pre- and post-RT in PNOC003 cohort. C and D, Kaplan–Meier PFS (C) and OS (D) curves after stratification of patients with (present) and without (absent) detectable plasma H3K27M-mutant ctDNA at baseline. VAF, variant allele frequency; ctDNA, circulating tumor DNA; RT, radiotherapy; PFS, progression-free survival; OS, overall survival. **, P < 0.01.