Figure 2.
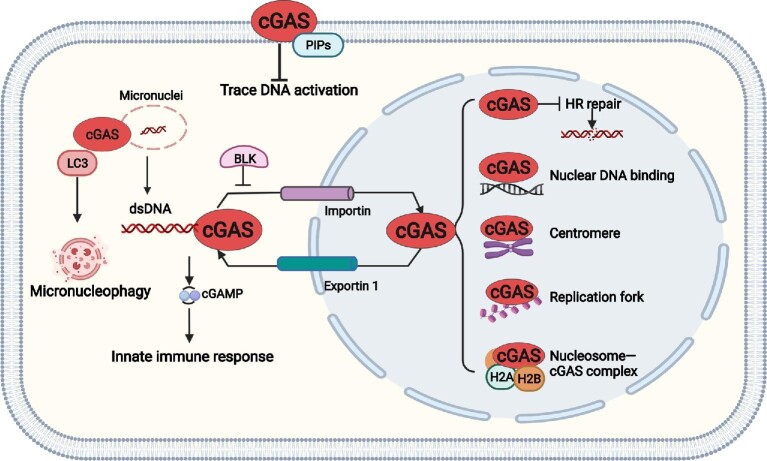
Regulation of cellular localization of cGAS. cGAS was originally found to be activated by dsDNA in the cytosol, which leads to the generation of the second messenger 2′3′-cGAMP and the induction of innate immune response. Now, it is generally accepted that cGAS is also located in the nucleus. Nuclear cGAS has been demonstrated to be critical in regulating DNA damage repair and replication fork stability. Nuclear cGAS is inactivated by association with nucleosome H2A and H2B. The property of chromosome binding and centromere location of cGAS has also been reported, though the functions remain elusive. In addition, the shuttle of cGAS between the cytosol and the nucleus is tightly regulated. NES is present in cGAS and mediates its translocation from the nucleus to the cytosol, which is critical for the DNA sensor function of cytosolic cGAS. NLS is also present in cGAS and its interaction with Importin is critical for the nuclear translocation of cGAS under genotoxic stresses. Moreover, the protein tyrosine kinase BLK-mediated phosphorylation of cGAS on tyrosine 215 is critical for its cytosolic retention. The electrostatic effects between the negative charge of PIPs on the membrane and the positive charge of the N-terminal structural domain of cGAS are critical for the membrane localization of cGAS in immune cells, which avoids the activation of cGAS by its own trace DNA in resting state. cGAS has also been found to locate on micronuclei, which mediates the sensing of dsDNA from micronuclei upon rupture. In addition. cGAS induces autophagy and lysosomal degradation of micronuclei by directly interacting with ATG8/LC3 in a STING-independent manner, and thereby serves as a micronucleophagy receptor. Though the membrane rupture and DNA damage of micronuclei have been implicated to be important for the recruitment of cGAS to micronuclei, the key factors involved in regulating the recruitment process remain to be identified.