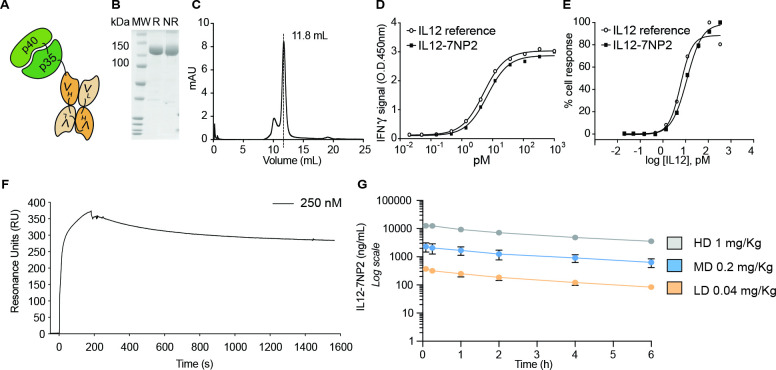
Figure 3.
In vitro characterization of IL12-7NP2 (A) Schematic representation of IL12-7NP2; (B) sodium dodecyl-sulfate polyacrylamide gel electrophoresis, 10% gel in non-reducing (NR) and reducing (R) conditions of purified IL12-7NP2; (C) size exclusion chromatogram of IL12-7NP2; (D–E) biological activity assay on human peripheral blood mononuclear cells (D) and on NK-92 cells (E) incubated with IL12-7NP2 and interleukin-12 (IL-12) reference standard; (F) surface plasmon resonance of IL12-7NP2 (250 nM) on streptavidin chip coated with human FAP (hFAP) (correction for change in the refraction index was applied); (G) pharmacokinetic analysis conducted in Cynomolgus monkeys injected once at the dose of 1 mg/kg, 0.2 mg/kg, or 0.04 mg/kg of IL12-7NP2. Blood samples were collected on day 1 and day 22 of the safety toxicology study, before dosing, at 5, 15, 60, 120, 240, and 360 min after end of dosing.