Abstract
Objective
In order to elucidate the biological activity of the co-cultured adventitious roots (ARs) of Echinacea pallida and Echinacea purpurea and provide theoretical basis for its application, and the anti-inflammatory activities and potential mechanisms of co-cultured ARs were studied.
Methods
The experimental materials were obtained by bioreactor co-culture technology and used in the activity research. In this study, mouse macrophages induced by lipopolysaccharide (LPS) were used as in vitro model. Different concentrations of AR extract (50–400 g/mL) were used to treat cells. The expression of pro-inflammatory cytokines was determined using enzyme linked immunosorbent assay. The inducible nitric oxide synthase and cyclooxygenase-2 expression, mitogen-activated protein kinase (MAPK) phosphorylation, and the inhibitor of nuclear factor-kappa B-α levels were determined by the Western blot analysis.
Results
In the co-cultured ARs, total flavonoids and total caffeic acid were determined, and the contents of both bioactive compounds were significantly higher than those ARs from the single-species culture. Compared with the control group, the large amount of pro-inflammatory mediators was released after LPS stimulation. However, in the extract groups with different concentrations (25, 50, and 100 g/mL), the production of these pro-inflammatory mediators was inhibited in a dose-dependent manner. Furthermore, the levels of phosphorylation of MAPK proteins, including p-p38, p-c-Jun N-terminal kinase, and p-extracellular regulated protein kinases were significantly (P < 0.05) decreased in the extract groups, revealing that the AR extract probably involved in regulating the MAPK signaling pathway.
Conclusion
Collectively, our findings suggested that the co-cultured ARs of E. pallida and E. purpurea can inhibit production of pro-inflammatory mediators in mouse peritoneal macrophages and possess the anti-inflammatory effect by regulating MAPK signaling pathways.
Keywords: adventitious root, anti-inflammation, co-culture, Echinacea pallida (Nutt.) Nutt, Echinacea purpurea (L.), Moench, mitogen-activated protein kinase
1. Introduction
Cellular agriculture science is an interdisciplinary branch combining biology and engineering, and is focused on the production of agriculture products from cell cultures (Stephens et al., 2018). In the early 20th century, cellular agriculture was firstly initiated in the commercial production of insulin and rennet (New Harvest, 2019), and recently has expanded to the large-scale production of valuable secondary metabolites from plants (Wang et al., 2017, He et al., 2018). As the key technology of the cellular agriculture, plant cell or organ culture can conveniently and effectively produce desired metabolites, thus, this technology has received global attention (Furusaki and Takeda, 2011). However, to date, the utilization of cultured plant cells or organs on the development as well as the production of products are insufficient, which one of the reasons is relatively lack of the evidence that the cultures possess similar bioactive property to their mother plants.
Echinacea pallida (Nutt.) Nutt. and Echinacea purpurea (L.) Moench are the most widely used medicinal Echinacea species (Gao et al., 2018). Their whole plants contain phenolics, flavonoids (including caffeic acid derivatives), polysaccharides, and other compounds (Pellati et al., 2004). Plants of both Echinacea species possess various bioactive properties such as antioxidative, antibacterial, antiviral, and antifungal properties, and they are often used to treat common cold and respiratory and urinary diseases (Barrett, 2003). However, the plant resource of Echinacea species cannot meet the market demand because of the instability of bioactive compound synthesis under natural environment, insufficient yield of the plant production, and other factors (Gao et al., 2018). Therefore, to relieve the pressure on the plant resource, seeking an alternative approach for obtaining the plant material is becoming a hotspot. At present, adventitious root (AR) culture has been systematically studied in numerous plant species, including E. pallida and E. purpurea (Gao et al., 2018, Hahn et al., 2009). Meanwhile, the co-culture technology has been applied in E. pallida and E. purpurea ARs, and the finding demonstrated that the bioactive compound synthesis was obviously increased, and some compounds undetected in the single-species culture were identified in the co-cultured ARs (Wu et al., 2017, Wu et al., 2018). Therefore, AR co-culture is favorable for the mass production of Echinacea bioactive compounds and has a potential as a novel plant material. To use ARs as the material of anti-inflammatory products, the present study determined effects of extracts from co-cultured ARs on the production of pro-inflammatory mediators, the phosphorylation of mitogen-activated protein kinase (MAPK) proteins, and the level of the inhibitor of nuclear factor-kappa B-α (IκB-α) in lipopolysaccharide (LPS)-induced mouse peritoneal macrophages (PMs) to clarify the anti-inflammatory property.
2. Materials and methods
2.1. Reagents
Indole-3-butyric acid (IBA) was obtained from Beijing Solarbio Science and Technology Co., Ltd., (Beijing, China). RIPA lysis buffer was purchased from Sigma (Sigma Aldrich Chemical Co., USA). Enzyme-linked immunosorbent assay (ELISA) Kits were purchased from BD Bioscience, (SD, USA). ATP was purchased from InvivoGen Co., (CA, USA).
2.2. Plant materials
ARs of E. pallida and E. purpurea were separately produced from bioreactors after 30 d of culture according to the method described by Wu et al. (Wu et al., 2012, Wu et al., 2007a). For AR co-culture, ARs (11.4 g) of E. pallida and E. purpurea (8.6 g) were cut into approximately 1 cm length and inoculated together into a 5 L air-lift bioreactor with 4 L working volume (Fig. 1). The culture medium was three-quarter of Murashige and Skoog (1962) medium supplemented with 1 mg/L IBA and 50 g/L sucrose. The pH of the medium was adjusted to 5.8 before sterilization at 121 °C and 1.2 kg/cm for 20 min. The bioreactors were maintained at (25 ± 2) °C in the dark, and aerated with sterile air at 100 mL/min. The co-cultured ARs were harvested after 35 d of bioreactor culture and washed with tap water thrice, then freeze-dried in a lyophilizer. The dry ARs were used to determine the bioactive compound contents and prepare the extract.
Fig. 1.
Structures of six caffeic acid derivatives (A) and HPLC chromatograms of standard and extract from co-cultured adventitious roots of E. pallida and E. purpurea (B). Peaks corresponding to caftaric acid (1), chlorogenic acid (2), caffeic acid (3), cynarine (4), echinacoside (5) and cichoric aicd (6), respectively.
2.3. Determination of bioactive compounds
The dry ARs of 0.2 g were soaked in 10 mL of 80% methanol for 10 min. The supernatant was collected after centrifugation and used to determine the bioactive compound contents. Total flavonoid content of ARs was quantitatively determined spectrophotometrically using a colorimetric method (Cui et al., 2010), with catechin as the standard. In brief, 0.25 mL of AR extract was added to 0.75 mL of 5% NaNO2 solution for 6 min of reaction; Then, 0.15 mL of 10% AlCl3 was added. Followed by adding with 1 mol/L NaOH. After 5 min, the reaction solution was measured at 510 nm of absorbance with a spectrophotometer (UV-2600, Shimadzu Corporation, Kyoto, Japan). The contents of caffeic acid derivative monomers were determined according to the method of Wu et al. (2007b). The caffeic acid fractions were analyzed using a high-performance liquid chromatography system equipped with XTerra RP 18 column (Fig. 2). The mobile phases were water (A) and acetonitrile (B). The gradient elution was modified as follows: initial 10% B for 40 min; 25% B for 11 min; 50% B for 1 min; with recycling to initial condition for 8 min at a flow rate of 0.3 mL/min. Caffeic acid derivatives were detected at 330 nm.
Fig. 2.
Adventitious roots of E. pallida and E. purpurea co-cultured in a bioreactor.
2.4. Extract preparation
ARs extract preparation using the method was described by Gao et al. (2019). The powdered ARs (30 g) were soaked in 400 mL methanol (80%) for 1 h, and filtered with a filter paper, then the residue was repeatedly extracted twice with methanol. All filtrates were merged and concentrated by using a vacuum rotary evaporator at 45 °C; The concentrated extract was lyophilized to obtain the extract.
2.5. Animals and ethical statement
C57BL/6 mice with a mean weight of 22–25 g were obtained from Changchun Yisi Experimental Animal Co., Ltd., (Changchun, China). All the experiments followed the guidelines established by the Institutional Animal Ethical Committee of Shahid Beheshti University of Medical Sciences, Tehran, Iran.
2.6. Cytotoxicity test
The PMs were obtained from C57BL/6 mice and the cells were cultured in the Dulbecco’s Modified Eagle Medium (DMEM) using the method described by Han et al. (2018). The extract was diluted into 0, 50, 100, 200, and 400 μg/L, and then the effect of extract concentrations on cell viability was determined to evaluate the cytotoxicity. The cell viability was determined by the method of (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (Mosmann, 1983).
2.7. Anti-inflammatory experiment design
The in vitro experimental model of LPS-induced inflammation was used in the present study. AR extract concentrations of 25, 50, and 100 μg/mL were selected depending on the result of the cytotoxic test, and the experimental groups were designed as follows: (1) control group (Cont), cells were not treated with an extract or LPS; (2) LPS group (LPS), cells were treated only with LPS (0.1 μg/mL); (3) Low-(E25), medium- (E50), and high- (E100) dose extract groups, cells were pretreated with 25, 50, and 100 μg/mL extracts, respectively, before LPS (0.1 μg/mL) stimulation for several times. The production of nitric oxide (NO), prostaglandin E2 (PGE2), tumor necrosis factor (TNF)-α, and interleukin (IL)-6 or −1β, and the expression of inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) in cells were determined to evaluate the anti-inflammatory activity. The phosphorylation of MAPKs, including p38 MAPK (p38), c-Jun N-terminal kinase (JNK), and extracellular regulated protein kinases (ERK) were investigated and the level of IκB-α was determined to clarify the molecular mechanism of anti-inflammation for the co-cultured Echinacea ARs.
2.8. Determination of NO and PGE2 production
Cells were seeded in 48-well plates (2.5 × 105 cells/well) containing DMEM supplemented with 10% FBS and 1% antibiotics (penicillin and streptomycin, 100 U/mL) and treated with AR extract for the following different time periods: 24 h for NO determination and 16 h for PGE2 determination; Followed by LPS (0.1 μg/mL) treatment for 1 h, the culture supernatant was harvested, and the NO level was measured according to the Griess reaction (Dirsch et al., 1998) with the optical density at 550 nm. The PGE2 level was determined via the ELISA assay using the Kit according to the manufacturer’s instructions, and the optical density was measured at 450 nm.
2.9. Determination of TNF-α, IL-6 and IL-1β production
The ELISA method was used to determine the production of TNF-α, IL-6, and IL-1β. The cells were seeded in 48-well plates (2.5 × 105 cells/well) for 12 h, and the extract was added. After 1 h of incubation, LPS (0.1 μg/mL) was added and stimulated for 6 h to examine the production of IL-6 and TNF-α. For determining the IL-1β production, cells were treated with the extract for 1 h after 3 h of LPS (0.1 μg/mL), then 5 mmol/L ATP was added for 1 h to prime cells for IL-1β maturation. TNF-α, IL-6, and IL-1β levels were determined via the ELISA assay using their relevant ELISA Kits according to the manufacturer’s instructions, and the optical densities were measured at 450 nm.
2.10. Determination of iNOS and COX-2 expression, MAPK phosphorylation, and IκB-α levels
The iNOS and COX-2 expression, MAPK phosphorylation, and IκB-α levels were determined by using the Western blot analysis. In brief, cells were seeded in 6-well plates (2.5 × 106 cells/well) for 12 h, treated with the AR extract for 1 h, and stimulated by LPS (0.1 μg/mL) for various times, those are 24 h for iNOS and COX-2 determination, 30 min for MAPK determination, and 15 min for IκB-α determination, then the medium of the cell plate was discarded, the RIPA lysis buffer was added, and the lysate was used for Western blot analysis (Martinon et al., 2002).
2.11. Experimental design and data analysis
Data were collected from three experimental replicates. The results are presented as mean ± standard error. The mean values were subjected to Duncan’s multiple-range test by the Statistical Analysis System (SAS) program with P < 0.05.
3. Results
3.1. Bioactive compounds in ARs
The ARs of E. pallida (11.4 g) and E. purpurea (8.6 g) were inoculated together in a 5 L bioreactors, and approximately 370 g (fresh weight) of ARs were obtained after 35 d of culture (Fig. 1). In the co-cultured ARs, 34.43 mg/g dry weight (DW) of total flavonoids and 26.79 mg/g DW of total caffeic acid derivatives were determined, and those contents were significantly higher than those in ARs from the single-species culture (Table 1). Meanwhile, the six monomers of caffeic acid derivatives were identified in co-cultured ARs, but some monomers were not identified in ARs from the single-species culture, such as caftaric acid and caffeic acid in E. pallida ARs, and caffeic acid in E. purperea ARs. Table 1 also showed that the co-cultured ARs contained maximum cichoric acid (13.48 mg/g DW), followed by chlorogenic acid (8.15 mg/g DW), and echinacoside (3.82 mg/g DW). On the contrary, relatively small amounts of caftaric acid (0.88 mg/g DW), caffeic acid (0.12 mg/g DW), and cynarine (0.34 mg/g DW) were found.
Table 1.
Bioactive compound contents in E. pallida, E. purpuerea, and their co-cultured adventitious roots.
| Bioactive compounds | Content (mg·g−1 DW) |
||
|---|---|---|---|
| E. pallida | E. purpuerea | Co-cultured Echinacea | |
| Total flavonoids | 19.80 ± 1.04c | 25.42 ± 1.51b | 34.43 ± 1.03 a |
| Total caffeic acid derivatives | 13.75 ± 0.36c | 19.51 ± 0.51b | 26.79 ± 0.65 a |
| Caftaric acid | − | 0.95 ± 0.06 a | 0.88 ± 0.03 a |
| Chlorogenic acid | 2.71 ± 0.07c | 5.45 ± 0.14b | 8.15 ± 0.17 a |
| Caffeic acid | − | − | 0.12 ± 0.01 |
| Cynarine | 0.51 ± 0.01 a | 0.33 ± 0.01b | 0.34 ± 0.01b |
| Echinacoside | 7.41 ± 0.19 a | 0.91 ± 0.02c | 3.82 ± 0.10b |
| Cichoric acid | 3.12 ± 0.08c | 11.87 ± 0.31b | 13.48 ± 0.35 a |
Note: The total flavonoid content was determined by spectrophotometry and caffeic acid derivatives were determined by HPLC. Total caffeic acid derivatives = caftaric acid + chlorogenic acid + caffeic acid + cynarine + echinacoside + cichoric acid. Data represents mean ± standard error (n = 3). The different letters within the same row indicate significant difference by Duncan’s multiple range test at 5% level.
3.2. Effect of AR extract on production of NO and PEG2
The cytotoxicity of the co-cultured AR extract was evaluated by determination of the cell viability. Fig. 3 showed that the cell viability after the extract treatment within their tested concentrations (50–400 μg/mL) did not decrease. Thus, further experiments used 25, 50, and 100 μg/mL of extracts to treat cells before LPS stimulation, and then determined the production of pro-inflammatory mediators.
Fig. 3.
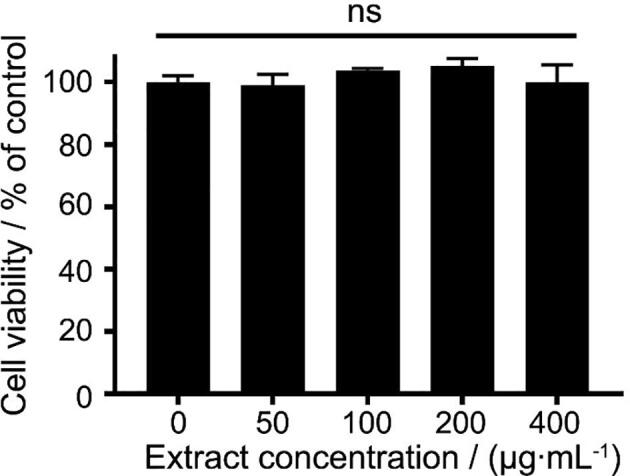
Effect of different concentrations of extracts from co-cultured Echinacea adventitious roots on cell viability. Data are presented as mean ± standard error (n = 3). Ns indicates no significant difference according to Duncan’s multiple range test at 5% level.
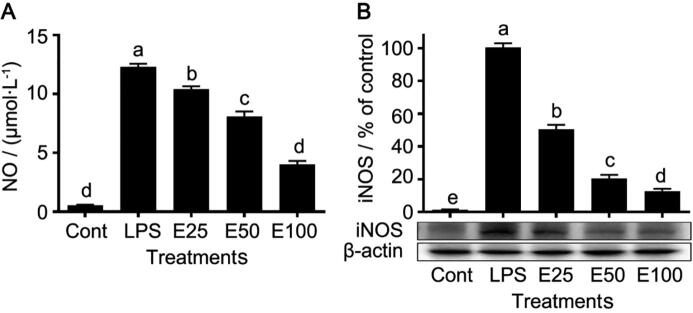
After LPS treatment, cells released large amounts of NO, and the NO level in the LPS group was 22.3-fold higher than that in the control group (Fig. 4A). However, NO levels in the low- (E25), middle- (E50), and high-dose (E100) extract groups were significantly (P < 0.05) lower than those in the LPS group, illustrating that the NO production was inhibited by the pretreatment of AR extracts, and the inhibitory effect exerted in a dose-dependent manner. The pattern of iNOS expression was similar to that of the NO production, and iNOS expression was significantly (P < 0.05) decreased in the extract groups (Fig. 4B).
Fig. 4.
Effect of extracts from co-cultured Echinacea adventitious roots on NO production (A) and iNOS expression (B) in cells. Data are presented as mean ± standard error (n = 3). The different letters between groups indicate significant difference by Duncan’s multiple range test at 5% level.
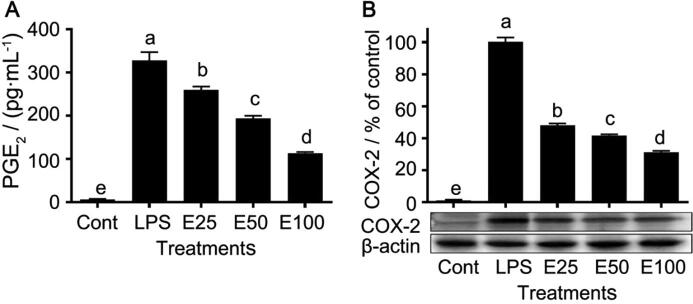
The PGE2 production in all extract groups (E25, E50, and E100) was significantly (P < 0.05) reduced compared with the LPS group, which was exerted in a dose-dependent manner (Fig. 5A). In addition, the expression of COX-2, an enzyme catalyzing PGE2 biosynthesis, was assayed. Fig. 5B exhibited that the expression level of COX-2 was consistent with the result of PGE2 production, which was decreased in the extract groups in a dose-dependent manner.
Fig. 5.
Effect of extracts from co-cultured Echinacea adventitious roots on PGE2 production (A) and COX-2 expression (B) in cells. Data are presented as mean ± standard error (n = 3). The different letters between groups indicate significant difference by Duncan’s multiple range test at 5% level.
3.3. Effect of AR extract on production of pro-inflammatory cytokines
Effect of AR extracts on the production of pro-inflammatory cytokines (IL-6 and −1β, and TNF-α) was also determined in the present study. The level of IL-6 dramatically (P < 0.05) was increased in the LPS group, but that was lower in the E50 or E100 group (Fig. 6A), revealing that the IL-6 production was inhibited by the pretreatment of AR extracts. The effects of different concentrations of AR extracts on the IL-1β production (Fig. 6B) were similar with that of the TNF-α (Fig. 6C). The levels of IL-1β and TNF-α were significantly (P < 0.05) lower in all extract groups (E25, E50, and E100) compared with those in the LPS group, and decreased in dose-dependent manner.
Fig. 6.
Effect of extracts from co-cultured Echinacea adventitious roots on production of pro-inflammatory cytokines of IL-6 (A), IL-1β (B), and TNF-α (C) in cells. Data are presented as the mean ± standard error (n = 3). The different letters between groups indicate significant difference by Duncan’s multiple range test at 5% level.
3.4. Effect of AR extract on MAPK phosphorylation and IκB-α level
In the present study, we investigated the effect of AR extracts on the MAPK phosphorylation and IκB-α level to understand whether the AR extract acts the anti-inflammatory effect through regulating the MAPK and nuclear factor-kappaB (NF-κB) signaling pathways. Fig. 7 showed that LPS enhanced the phosphorylation of MAPK proteins, thus, the p-p38 (Fig. 7A), p-JNK (Fig. 7B), and p-ERK (Fig. 7C) levels were remarkably (P < 0.05) higher in the LPS group than the extract groups, and they were dose-dependently reduced in all extract groups (E25, E50, and E100). In addition, LPS stimulation accelerated the IκB-α level, however, which was not affected by the AR extracts (Fig. 8).
Fig. 7.
Effect of extracts from co-cultured Echinacea adventitious roots on production of phosphorylation of MAPKs in cells. Data are presented as the mean ± standard error (n = 3). The different letters between groups indicate significant difference by Duncan’s multiple range test at 5% level.
Fig. 8.

Effect of extracts from co-cultured Echinacea adventitious roots on IκB-α level in cells. Data are presented as the mean ± standard error (n = 3). The different letters between groups indicate significant difference by Duncan’s multiple range test at 5% level.
4. Discussion
Caffeic acid derivatives, including caftaric acid, caffeic acid, chlorogenic acid, cynarin, echinacoside, and cichoric acid, belong to flavonoids, which are the main bioactive compounds in Echinacea plants (Cui et al., 2013). Studies have indicated that caffeic acid derivatives contained in Echinacea AR cultures, and their contents were higher in ARs than field-grown plants (Wu et al., 2012), and those in ARs from co-culture of E. pallida and E. purpurea were higher compared with the single-species culture (Wu et al., 2017). Caffeic acid derivatives have been proved having good anti-inflammatory effects. For instance, Cunha et al. (2004) found that caffeic acid derivatives from the reaction of caffeic acid inhibited the NO production and iNOS expression in LPS-induced Raw 264.7 macrophages. It was suggested that caffeic acid derivatives from Salvia miltiorrhiza roots may have the anti-inflammatory activities (Choi et al., 2017). Therefore, the present study investigated the anti-inflammation effect of co-culture Echinacea ARs for providing an evidence to the product production using as the plant material or additives.
LPS stimulation can induce macrophages to produce inflammatory mediators. As a small toxic molecule, NO is generated by a group of cytosolic or membrane-bound isoenzymes (Panaro et al., 2003), and iNOS is the cytokine-inducible isoform of the nitric oxide synthase (Murakami and Ohigash, 2010). In addition, COX-2 is an inducible enzyme, which is frequently overexpressed during inflammation, and PGE2 synthesis is regulated by COX-2 (Tian et al., 2019). Thus, the excessive production of NO and PGE2 can be prevented through modulating iNOS and COX-2 expression, respectively, which is important for the development of pharmacological strategies against inflammation. Furthermore, the overproduction of pro-inflammatory cytokines may accelerate the pathological changes (Chen et al., 2019). In the present study, the NO or PGE2 production was inhibited by the pretreatment with AR extracts, attributing to the strong inhibition on the expression of iNOS or COX-2. Meanwhile, AR extracts significantly (P < 0.05) inhibited the IL-6, IL-1β, and TNF-α production and the levels of those pre-inflammatory cytokines were decreased in a dosage-dependent manner. Our findings confirmed that co-cultured Echinacea ARs can inhibit the LPS-induced inflammation, indicating an applicable potential of ARs in the production of anti-inflammatory products.
The cell-membrane receptor (toll-like receptor 4, TLR4) recognizes LPS after LPS stimulation and activates different signaling pathways, such as myeloid differentiation factor 88 (MyD88)-dependent and -independent pathways (He et al., 2013). The downstream signaling pathways in the MyD88 dependent pathway, including MAPK and NF-κB pathways, are initiated when the intercellular signaling cascade is triggered by TLR4, and the secretion of pro-inflammatory factors are further elevated (Doz et al., 2009). In the MAPK signaling pathway, MAPK proteins such as p38, JNK, and ERK are phosphorylated and modulate transcription factors, resulting in various biological responses including inflammation (Marie & Roux, 2011). The activation of NF-κB in the NF-κB pathway is initiated by the phosphorylation of the IκB-α protein. The IκB-α phosphorylation allows the NF-κB complex to be freed and entered the nucleus to trigger the expression of specific genes, leading to the physiological response (Deptala et al., 2015). The present study found that AR extracts inhibited the MAPK phosphorylation, but did not affect the IκB-α level, indicating that AR extracts involved in regulating the MAPK signaling pathway rather than the NF-κB signaling pathway.
To date, the anti-inflammatory effect has been repeatedly reported in various Echinacea species (Raso et al., 2010, Speroni et al., 2002, Tubaro et al., 2011), and the associated products have also been sold in the international market. Along with the increase of the market demand, the supply of Echinacea raw material is seriously inadequate worldwide. Thus, AR cultures become an alternative supply source and have a great potential to be used in the industrial production.
5. Conclusion
The extract of co-cultured Echinacea ARs inhibited the production of various pro-inflammatory mediators in PMs, and the anti-inflammatory effect of the extract was attributed to the regulation of MAPK signaling pathway. The findings of the present study suggested that the co-cultured Echinacea ARs possess a potential for using as the raw plant material or additives in the production of anti-inflammatory related products.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
This work was supported by grants the Jilin Scientific and Technological Development Program (20180101278JC) for the financial support and the National Natural Science Foundation of China (31370388 and 31660080).
Contributor Information
Jun Jiang, Email: jiangjun@ybu.edu.cn.
Mei-lan Lian, Email: lianmeilan2001@163.com.
References
- Barrett B. Medicinal properties of Echinacea: A critical review. Phytomedicine. 2003;10(1):66–86. doi: 10.1078/094471103321648692. [DOI] [PubMed] [Google Scholar]
- Chen Q., Zhang K.X., Li T.Y., Piao X.M., Lian M.L., An R.B., et al. Cardamine komarovii flower extract reduces lipopolysaccharide induced acute lung injury by inhibiting MyD88/TRIF signaling pathways. Chinese Journal of Natural Medicines. 2019;17(6):461–468. doi: 10.1016/S1875-5364(19)30053-6. [DOI] [PubMed] [Google Scholar]
- Choi H.G., Tran P.T., Lee J.H., Min B.S., Kim J.A. Anti-inflammatory activity of caffeic acid derivatives isolated from the roots of Salvia miltiorrhiza. Archives of Pharmacal Research. 2017;41(1):1–7. doi: 10.1007/s12272-017-0983-1. [DOI] [PubMed] [Google Scholar]
- Cui H.-Y., Abdullahil Baque M.d., Lee E.-J., Paek K.-Y. Scale-up of adventitious root cultures of Echinacea angustifolia in a pilot-scale bioreactor for the production of biomass and caffeic acid derivatives. Plant Biotechnology Reports. 2013;7(3):297–308. [Google Scholar]
- Cui X.-H., Chakrabarty D., Lee E.-J., Paek K.-Y. Production of adventitious roots and secondary metabolites by Hypericum perforatum L. in a bioreactor. Bioresource Technology. 2010;101(12):4708–4716. doi: 10.1016/j.biortech.2010.01.115. [DOI] [PubMed] [Google Scholar]
- da Cunha F.M., Duma D., Assreuy J., Buzzi F.C., Niero R., Campos M.M., et al. Caffeic acid derivatives: In vitro and in vivo anti-inflammatory properties. Free Radical Research. 2004;38(11):1241–1253. doi: 10.1080/10715760400016139. [DOI] [PubMed] [Google Scholar]
- Deptala A., Bedner E., Gorczyca W., Darzynkiewicz Z. Activation of nuclear factor kappa B (NF-kappaB) assayed by laser scanning cytometry (LSC) Cytometry Part B-Clinical. Cytometry. 2015;33:376–382. doi: 10.1002/(sici)1097-0320(19981101)33:3<376::aid-cyto13>3.0.co;2-q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirsch V., Stuppner H., Vollmar A. The Griess assay: Suitable for a bioguided fractionation of anti-inflammatory plant extracts. Planta Medica. 1998;64(05):423–426. doi: 10.1055/s-2006-957473. [DOI] [PubMed] [Google Scholar]
- Doz E., Rose S., Court N., Front S., Vasseur V., Charron S., et al. Mycobacterial phosphatidylinositol mannosides negatively regulate host toll-like receptor 4, MyD88-dependent proinflammatory cytokines, and TRIF-dependent co-stimulatory molecule expression. Journal of Biological Chemistry. 2009;284(35):23187–23196. doi: 10.1074/jbc.M109.037846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusaki S., Takeda T. Bioreactors for plant cell culture. Plant Cell Biotechnology. 2011;2:361–372. [Google Scholar]
- Gao Y., Wu C.H., Piao X.C., Han L., Gao R., Lian M.L. Optimization of culture medium components and culture period for production of adventitious roots of Echinacea pallida (Nutt.) Nutt. Plant Cell Tissue & Organ Culture. 2018;135(2):299–307. [Google Scholar]
- Gao Y., Xiu J.R., Tian W., Piao X.C., Lian M.L. Adventitious roots of Echinacea pallida inhibit the production of pro-inflammatory mediators in LPS induced mouse macrophages. Journal of Agricultural Science Yanbian University. 2019;41(2):1–8. [Google Scholar]
- Hahn E.J., Wu C.H., Paek K.Y. Production of root biomass and secondary metabolites through adventitious root cultures of Echinacea purpurea in bioreactors. Acta Horticulturae. 2009;(829):73–78. doi: 10.17660/ActaHortic.2009.829.9. [DOI] [Google Scholar]
- Han X.-Z., Ma R., Chen Q.i., Jin X., Jin Y.-Z., An R.-B., et al. Anti-inflammatory action of Athyrium multidentatum extract suppresses the LPS-induced TLR4 signaling pathway. Journal of Ethnopharmacology. 2018;217:220–227. doi: 10.1016/j.jep.2018.02.031. [DOI] [PubMed] [Google Scholar]
- He W., Qu T., Yu Q., Wang Z., Lv H., Zhang J., et al. LPS induces IL-8 expression through TLR4, MyD88, NF-kappaB and MAPK pathways in human dental pulp stem cells. International Endodontic Journal. 2013;46(2):128–136. doi: 10.1111/j.1365-2591.2012.02096.x. [DOI] [PubMed] [Google Scholar]
- He X.P., Su D.Q., Ding L.J., He S. Secondary metabolites of co-culture of Alternaria alternate YX-25 and Streptomyces exfoliatus YX-32. Chinese Traditional and Herbal Drugs. 2018;49(24):5772–5779. [Google Scholar]
- Marie C., Roux P.P. Activation and function of the MAPKs and their substrates, the MAPK-activated protein kinases. Microbiology and Molecular Biology Reviews. 2011;75:50–83. doi: 10.1128/MMBR.00031-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinon F., Burns K., Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proil-beta. Molecular Cell. 2002;10(2):417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- Murakami A., Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: Chemoprevention using food phytochemicals. International Journal of Cancer. 2010;121(11):2357–2363. doi: 10.1002/ijc.23161. [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. A revised medium for rapid growth and bioassay with tobacco tissue cultures. Physiology Plantarum. 1962;15:473–497. [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. Journal of Immunological Methods. 1983;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- New Harvest. What is cellular agriculture, [EB/OL]. https://www.new-harvest.org/cell_ag_101. 2019-11-29.
- Panaro M.A., Brandonisio O., Acquafredda A., Sisto M., Mitolo V. Evidences for iNOS expression and nitric oxide production in the human macrophages. Current drug targets – immune. Endocrine & Metabolic Disorders. 2003;3(3):210–221. doi: 10.2174/1568008033340216. [DOI] [PubMed] [Google Scholar]
- Pellati F., Benvenuti S., Magro L., Melegari M., Soragni F. Analysis of phenolic compounds and radical scavenging activity of Echinacea spp. Journal of Pharmaceutical & Biomedical Analysis. 2004;35(2):289–301. doi: 10.1016/S0731-7085(03)00645-9. [DOI] [PubMed] [Google Scholar]
- Raso G.M., Pacilio M., Carlo G.D., Esposito E., Pinto L., Meli R. In-vivo and in-vitro anti-inflammatory effect of Echinacea purpurea and Hypericum perforatum. Journal of Pharmacy & Pharmacology. 2010;54(10):1379–1383. doi: 10.1211/002235702760345464. [DOI] [PubMed] [Google Scholar]
- Speroni E., Govoni P., Guizzardi S., Renzulli C., Guerra M.C. Anti-inflammatory and cicatrizing activity of Echinacea Pallida Nutt root extract. Journal of Ethnopharmacology. 2002;79(2):265–272. doi: 10.1016/s0378-8741(01)00391-9. [DOI] [PubMed] [Google Scholar]
- Stephens N., Di Silvio L., Dunsford I., Ellis M., Glencross A., Sexton A. Bringing cultured meat to market: Technical, socio-political, and regulatory challenges in cellular agriculture. Trends in Food Science & Technology. 2018;78:155–166. doi: 10.1016/j.tifs.2018.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W., Piao X.-M., Yin C.-R., Jiang X.-L., Sun H.-D., An X.-L., et al. Adventitious root cultures of Oplopanax elatus inhibit LPS-induced inflammation via suppressing MAPK and NF-κB signaling pathways. Vitro Cellular & Developmental Biology-Animal. 2019;55(9):766–775. doi: 10.1007/s11626-019-00396-7. [DOI] [PubMed] [Google Scholar]
- Tubaro A., Tragni E., Del N.P., Galli C.L., Della L.R. Anti-inflammatory activity of a polysaccharidic fraction of Echinacea angustifolia. Journal of Pharmacy & Pharmacology. 2011;39(7):567–569. doi: 10.1111/j.2042-7158.1987.tb03182.x. [DOI] [PubMed] [Google Scholar]
- Wang J., Li J.-l., Li J., Li J.-X., Liu S.-J., Huang L.-q., et al. Production of active compounds in medicinal plants: From plant tissue culture to biosynthesis. Chinese Herbal Medicines. 2017;9(2):115–125. [Google Scholar]
- Wu C.H., An D., Sun L.N., Wang M., Chang G.N., Zhao C.Y., et al. A novel co-culture system of adventitious roots of Echinacea species in bioreactors for high production of bioactive compounds. Plant Cell Tissue & Organ Culture. 2017;130(2):301–311. [Google Scholar]
- Wu C.H., Hahn E.J., Paek K.Y. Biomass accumulation and bioactive compound production from adventitious root cultures of Echinacea purpurea in bioreactors. Journal of Biological Chemistry. 2007;259(22):14282–14285. [Google Scholar]
- Wu C.H., Huang T., Cui X.H., Paek K.Y. Induction of adventitious roots of Echinacea pallida and accumulation of caffeic acid derivatives. China Journal of Chinese Materia Medica. 2012;37(24):68–72. [PubMed] [Google Scholar]
- Wu C.H., Murthy H., Hahn E.Y., Paek K.Y. Large-scale cultivation of adventitious roots of Echinacea purpurea in airlift bioreactors for the production of cichoric acid, chlorogenic acid and cafteric acid. Biotechnology Letters. 2007;29:1179–1182. doi: 10.1007/s10529-007-9399-1. [DOI] [PubMed] [Google Scholar]
- Wu C.H., Tang J., Jin Z.X., Wang M., Liu Z.Q., Huang T., et al. Optimizing co-culture conditions of adventitious roots of Echinacea pallida and Echinacea purpurea in air-lift bioreactor systems. Biochemical Engineering Journal. 2018;132:206–216. [Google Scholar]