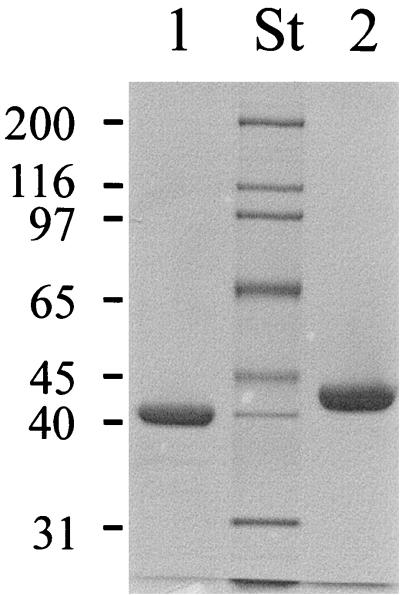
FIG. 4.
SDS gel of purified MalEAa. Wild-type and recombinant MalEAa proteins were purified from the culture supernatant of A. acidocaldarius cells and from the cytosol of E. coli strain ED169(pAH18), respectively, by affinity chromatography through agarose-coupled amylose. Aliquots were subjected to SDS-polyacrylamide gel electrophoresis, and the gel was subsequently stained with Coomassie brilliant blue. Lane 1, wild-type MalEAa (2.5 μg); lane 2, recombinant MalEAa (5 μg); lane St, molecular weight standards (in thousands). The observed difference in molecular weight is due to exposure of the wild-type protein to an extracellular protease that clips 23 amino acids from the N terminus (24).