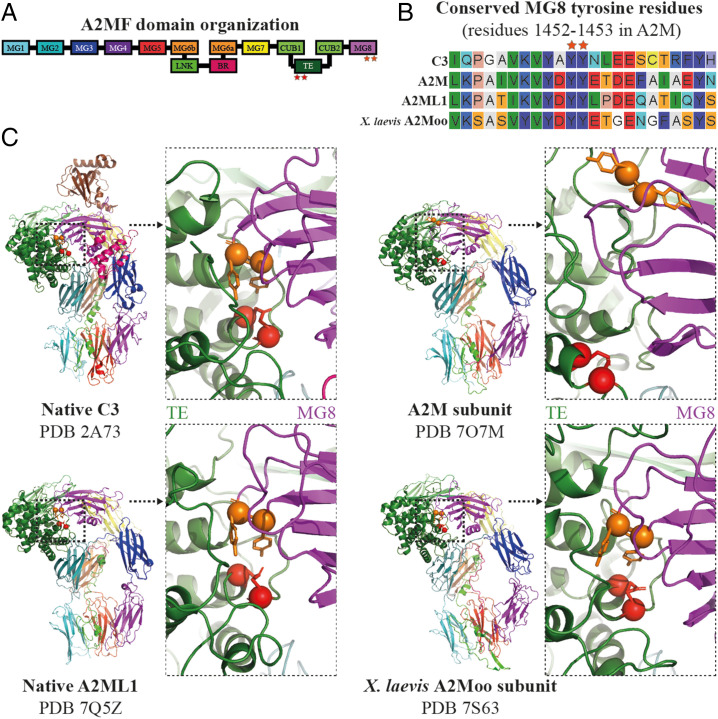
Fig. 1.
Comparison of the TE and MG8 domain interface in A2MF structures. (A) A schematic illustration of the domain organization of a typical A2MF protein. (B) Sequence alignment of human C3, A2M, and A2ML1, as well as A2Moo from the frog X. laevis in the vicinity of the conserved tyrosine residues in the MG8 domain. The tyrosine residues are indicated with orange stars and are highly conserved. (C) Cartoon representations of the structures of native C3 (PDB 2A73), an A2M subunit (PDB 7O7M), native A2ML1 (PDB 7Q5Z), and a frog A2Moo subunit (PDB 7S63). The TE cysteine and glutamine residues are shown in red and the conserved MG8 tyrosine residues are shown in orange, all with sphere Cα and stick side-chain representations. Domains are colored as indicated in A. Each structure is shown in its entirety and zoomed in toward the TE/MG8 domain interface. In C3, A2ML1, and A2Moo the TE is closely shielded by the MG8 tyrosines, whereas in the A2M subunit the TE is distant from the tyrosines.