Fig. 1.
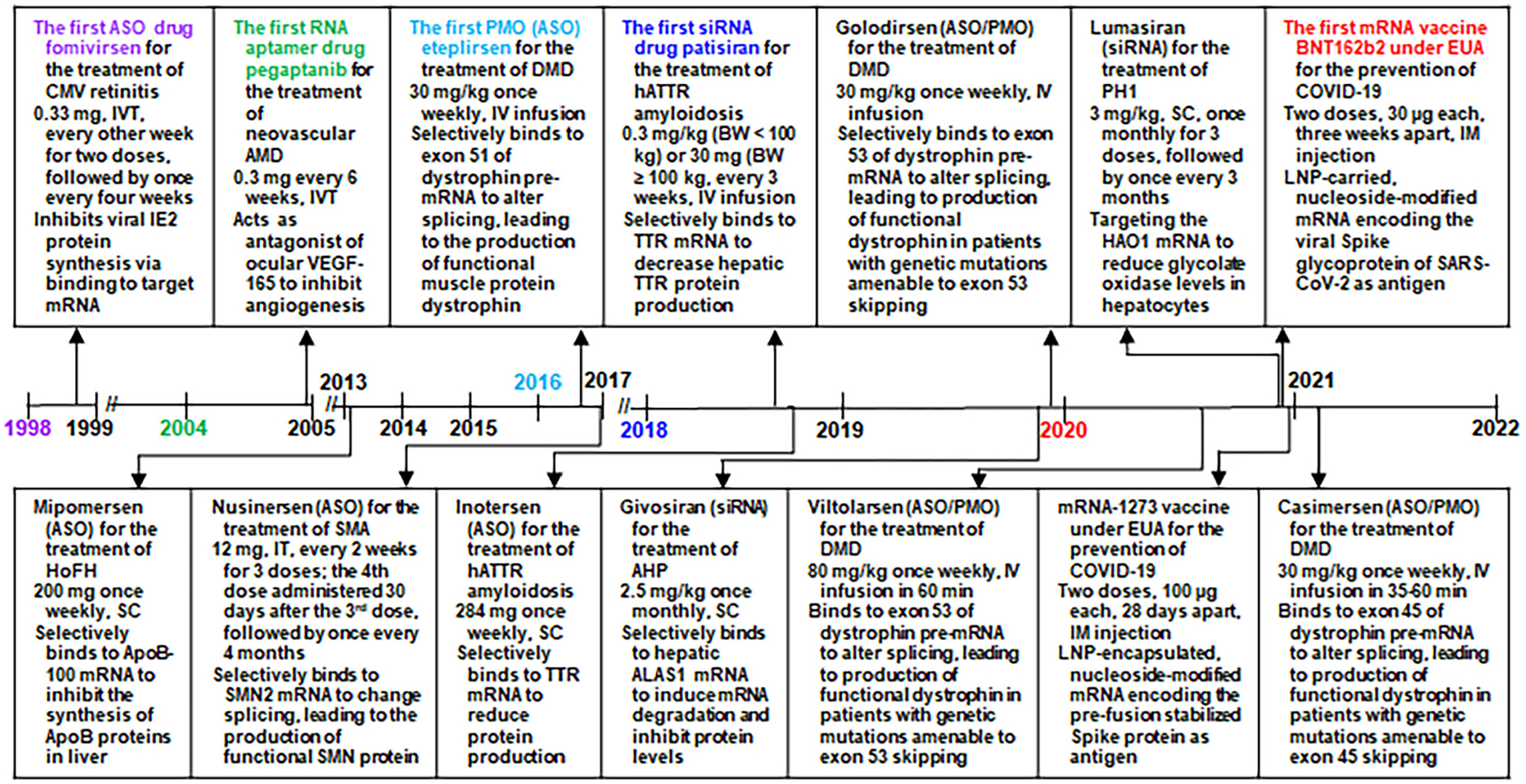
Timeline of the approval of RNA medications and vaccines by the US FDA and their indications, dosage regimens, and pharmacological actions. Note that (1) fomivirsen is an oligodeoxynucleotide acting as an ASO and it was withdrawn from the US market in 2006, (2) mipomersen was withdrawn from the US market in 2019, (3) the four PMO drugs consist of nucleobase thymine or 5-methyluracil, and (4) the two mRNA vaccines with FDA approval under emergency use authorization (EUA) are not for therapy but prevention from disease. AHP, acute hepatic porphyria; ALAS1, aminolevulinic acid synthase 1; AMD, age-related macular degeneration; ApoB, apolipoprotein B; ASO, antisense oligonucleotide; BW, body weight; COVID-19, coronavirus disease 2019; DMD, Duchenne muscular dystrophy; EUA, emergency use authorization; HAO1, hydroxyacid oxidase 1; hATTR amyloidosis, hereditary transthyretin-mediated amyloidosis; HoFH, homozygous familial hypercholesterolemia; IM, intramuscular; IV, intravenous; IVT, intravitreal injection; IT, intrathecally; LNP, lipid nanoparticle; PH1, primary hyperoxaluria type 1; PMO, phosphorodiamidate morpholino oligomer; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; SC, subcutaneous; siRNA, small interfering RNA; SMA, spinal muscular atrophy; SMN, survival motor neuron; TTR, transthyretin; VEGF, vascular endothelial growth factor.
