Fig. 3.
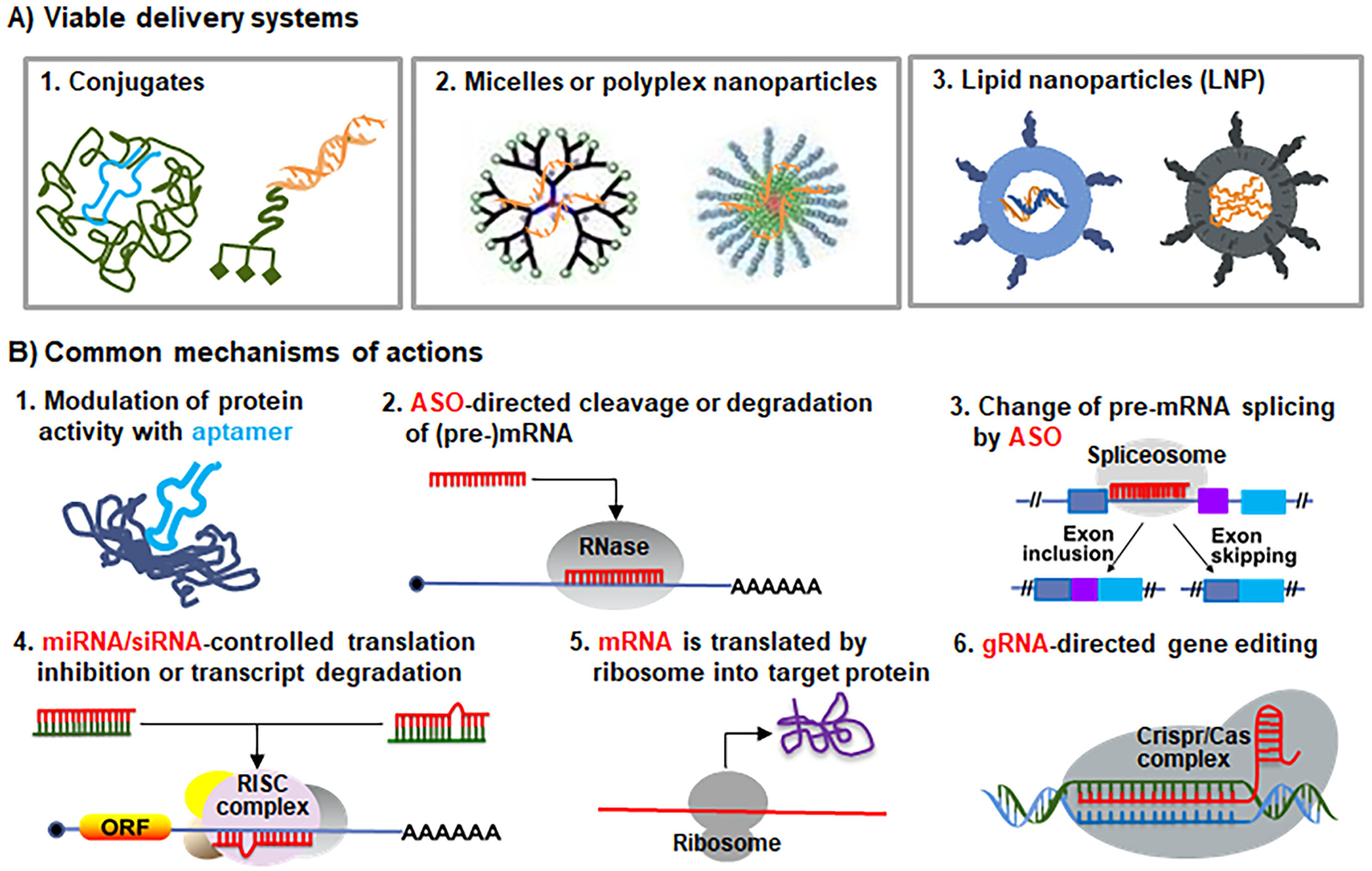
Common delivery systems and mechanisms of actions of therapeutic RNAs. (A) RNA modalities may be conjugated with specific polymers (e.g., PEG) or ligands (e.g., GalNAc), packaged with polymers (e.g., PEI), or formulation into lipid nanoparticles (e.g., using ionizable lipids) to improve the pharmacokinetics properties and enhance cellular uptake. See Figs. 4–5 for specific modifications used in RNA medications approved by the FDA. (B) Common mechanisms of actions of RNA therapeutics and vaccines marketed or under development. First, RNA aptamer drugs (e.g., pegaptanib) directly bind to extracellular, cell-surface or intracellular protein targets to block or activate their activities. Second, ASOs (e.g., DNA oligomer fomivirsen and “Gapmer” mipomersen and inotersen) direct RNases such as RNase H to cleave and degrade target transcripts within nucleus or cytoplasm. Third, ASOs (e.g., nusinersen and the PMOs (eteplirsen, golodirsen, viltolarsen and casimersen)) target the splice sites of pre-mRNAs and interact with splicing factors to alter RNA maturation in nucleus, causing exon inclusion or skipping. Fourth, the antisense or guide strands of siRNAs or miRNA mimics (e.g., patisiran, givosiran and lumasiran) are loaded into the cytoplasmic RISC complex to recognize target transcripts, leading to translation inhibition, mRNA cleavage or deadenylation. Indeed, both patisiran and lumasiran were designed to act on the 3′-untranslated regions of target transcripts, same as endogenous miRNAs. Fifth, exogenous mRNAs use cellular ribosomes to synthesize target proteins for replacement therapy or vaccination. Sixth, gRNAs interact with target gene sequences within nuclear CRISPR/Cas complex to achieve genome editing.
