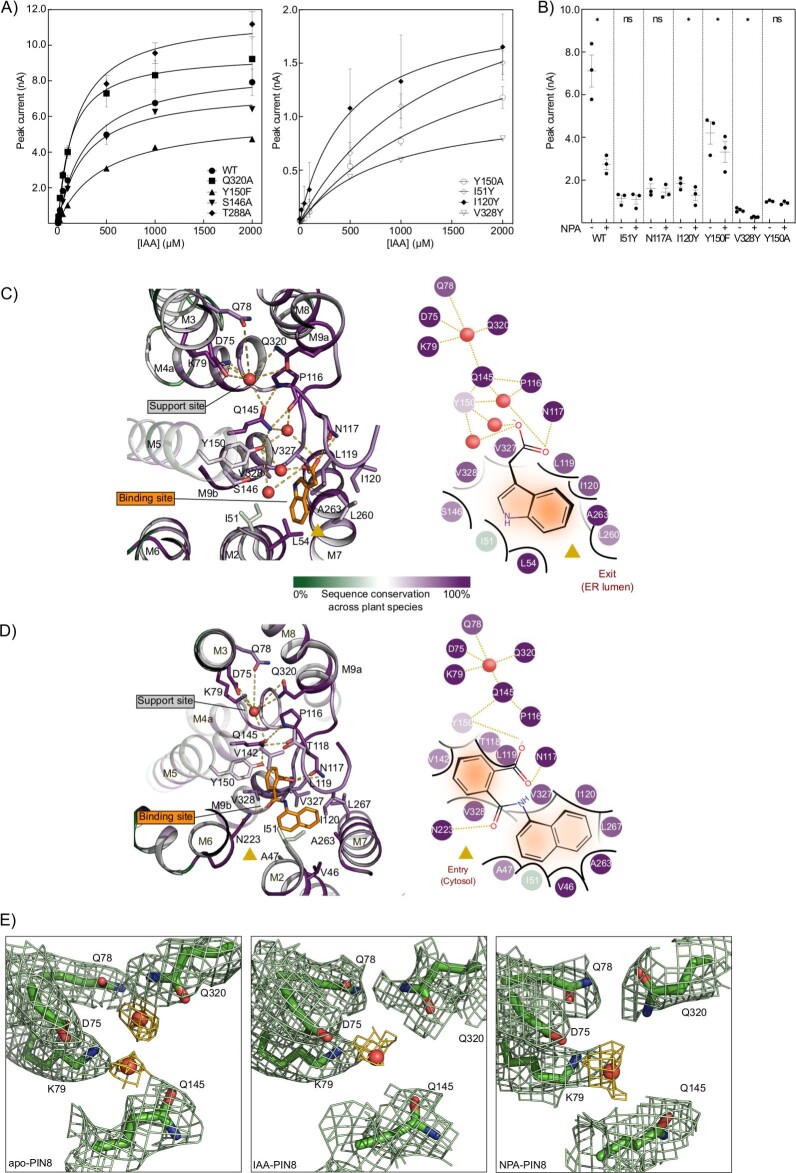
Extended Data Fig. 8. Details of mutants and support site.
A) Transport current using SSM-electrophysiology on PIN8 mutants in proteoliposomes. Transport can be described by Michaelis-Menten kinetics. Data points are mean or mean ± SE (n > 2)(WT n = 4 different liposome preparations, for mutants n = 5 (T288A), n = 4 (Q320A), n = 3 (I51Y, I120Y, Y150A), n = 2 (Y150F, S146A, V328Y). B) Sensitivity of WT and selected mutants to NPA inhibition. Peak current response to 2 mM IAA or 2 mM IAA and 20 µM NPA presented in non-activating as well as activating buffer. Asterisks indicate significant differences between groups (two-sided paired t-test, WT p = 0.0131, I51Y p = 0.48, N117A p = 0.07, I120Y p = 0.03, Y150F p = 0.02, V328Y p = 0.01, Y150A p = 0.22). Data points are mean ± SE; data points are individual experiments (n = 4 (V328Y), n = 3 (all other mutants and WT)). C) View from the non-cytosolic side of the side chains interacting with IAA and forming the support site. Residues are colored by sequence conservation using ConSurf. 318 unique sequences from plants with sequence identity of 35–95% to AtPIN8 were identified, sorted by E-value and 150 selected at equal intervals for the alignment. D) View from the non-cytosolic side of the side chains interacting with NPA and forming the support site. Residues are colored by sequence conservation using ConSurf. E) Map density for the peaks found in the support site modeled as water. In the case of apo-PIN two peaks could be modeled as water with one having stronger density than the other