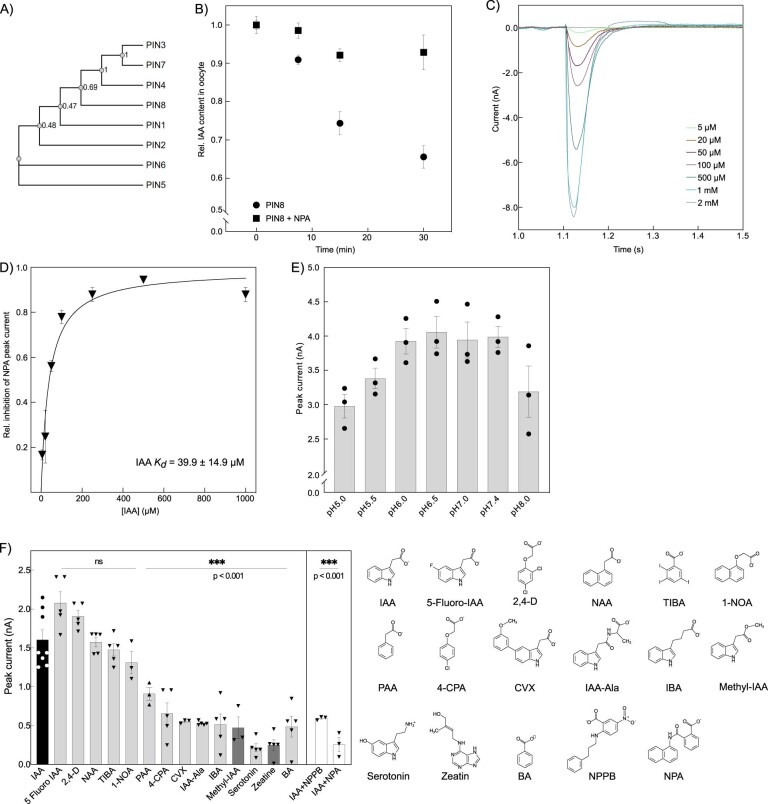
Extended Data Fig. 2. Functional data on PIN8.
A) Dendrogram of the relationship between Arabidopsis thaliana PIN1–8. Numbers denote bootstrap values of 500 trials. PIN8 is in a clade with the canonical PIN3, PIN4, and PIN7, unlike other non-canonical PINs (PIN5, PIN6). B) Figure time course of IAA export by PIN8 from oocytes. Relative IAA content of oocytes expressing PIN8 in the presence (■□) or absence (●) of 10 µM NPA internally determined at the time indicated after substrate injection. Initial internal IAA concentration was 1 µM. n = 10 oocytes at each time point. Data points are mean ± SE. C) Raw current traces from SSM-electrophysiology for the PIN8 WT proteoliposomes. D) Relative inhibition of the peak binding current induced by 100 µM NPA in the presence of the indicated IAA concentration in non-activating as well as activating buffer. Half-maximal inhibition 39.9 ± 14.9 µM (mean ± SE, n = 3) corresponds to apparent Kd(IAA). E) Peak currents elicited by 500 µM IAA at the pH indicated (n = 3). Bars are mean ± SE. The points represent individual measurements. F) Substrate specificity of PIN8 measured at pH 7.4. Peak currents elicited by IAA (●) or a range of putative substrates tested at 100 µM (▼). Synthetic auxins: 5-fluoro-IAA, 2,4-D, NAA, TIBA, 1-NOA, 4-CPA, CVX. Endogenous auxins: PAA, IAA-Ala, IBA, Methyl-IAA. Others: Serotonin, Zeatin (a cytokinin), BA (benzoic acid). Chemical structures at pH 7.4 are shown. Current response of substrates indicated with asterisks differed significantly from IAA, indicating that they are likely not substrates for the transporter, but we note that different chemical molecules have different electrostatic potentials and this can also have an influence on the observed current (5-Fluoro IAA p = 0.011; 2,4-D p = 0.272; NAA p = 0.999; TIBA p = 0.989; 1-NOA p = 0.539; PAA p = 0.0007, 4-CPA p < 0.0001; CVX p < 0.0001; IAA-Ala p < 0.0001; IBA p < 0.0001; Methyl-IAA p < 0.0001; Serotonin p < 0.0001; Zeatin p < 0.0001; BA p < 0.0001; IAA+NPPB p < 0.0001; IAA+NPA p < 0.0001). Substrates shown in dark grey are uncharged. Two inhibitors were tested in the presence of 100 µM IAA. Bars are mean ± SE; The data points represent individual measurements. (n = 8: IAA; n = 5: 5-Fluoro IAA, 2,4-D, NAA, TIBA, 4-CPA, IAA-Ala, IBA, Serotonin, Zeatin, BA; n = 3: 1-NOA, PAA, CVX, Methyl-IAA, IAA+NPPB, IAA+NPA)