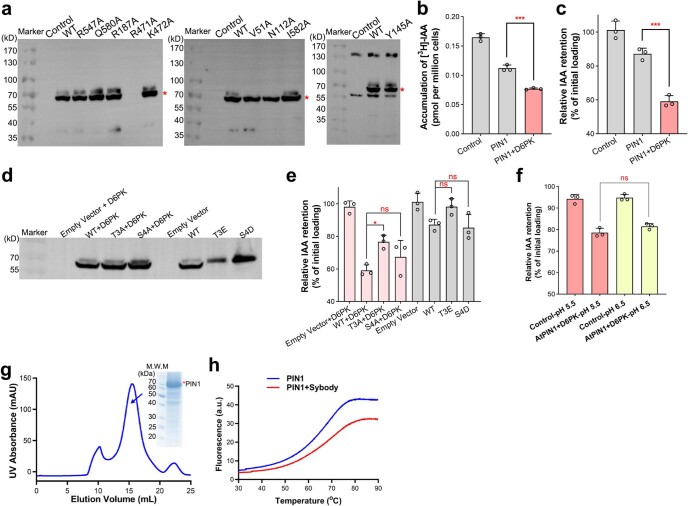
Extended Data Fig. 2. PIN1-mediated auxin transport in HEK293F cells.
a, PIN1 expression detected by whole-cell western blot using anti-Flag tag antibody. Independent experiments have been repeated for three times with similar results. b, Co-expression of PIN1 and D6PK further decreases [3H]-IAA accumulation in loading assay. c, Co-expression of PIN1 and D6PK further decreases [3H]-IAA retention in auxin efflux assay. Independent experiments were repeated three times for constructs in (b, c). Significances were determined using a two-tailed unpaired t-test. *** P = 0.0004 for AtPIN1 versus PIN1 plus D6PK in (b), *** P = 0.0005 for PIN1 versus PIN1 plus D6PK in (c). Data are presented as mean ± SD. d, Expressions of the PIN1 phosphosites mutants examined by western blot. T3E mutant had a relatively decreased expression while all other constructs have similar expression levels. e, Relative [3H]-IAA retention for the phosphosites mutants in efflux assay. Independent experiments were repeated three times for each construct. Significances were determined using a two-tailed unpaired t-test. * P = 0.0182, ns = not significant. Data are presented as mean ± SD. f, IAA efflux at different pH. After [3H]-IAA loading in pH 5.5 buffer, cells were transferred to a buffer with a pH of 5.5 or 6.5, respectively. n = 3 biologically independent experiments for each group. Significances were determined using a two-tailed unpaired t-test. ns = not significant. Data are presented as mean ± SD. g, Representative gel filtration and Coomassie-blue-staining SDS-PAGE results of WT PIN1 alone. Independent experiments have been repeated for three times with similar results. h, Microscale fluorescent based thermal stability assay result for the PIN1 protein alone, or with Sybody-21. The thermal stability of PIN1 was increased by ~5 degrees in the presence of Sybody-21. Full version of all gels and blots are provided in Supplementary Fig. 1.