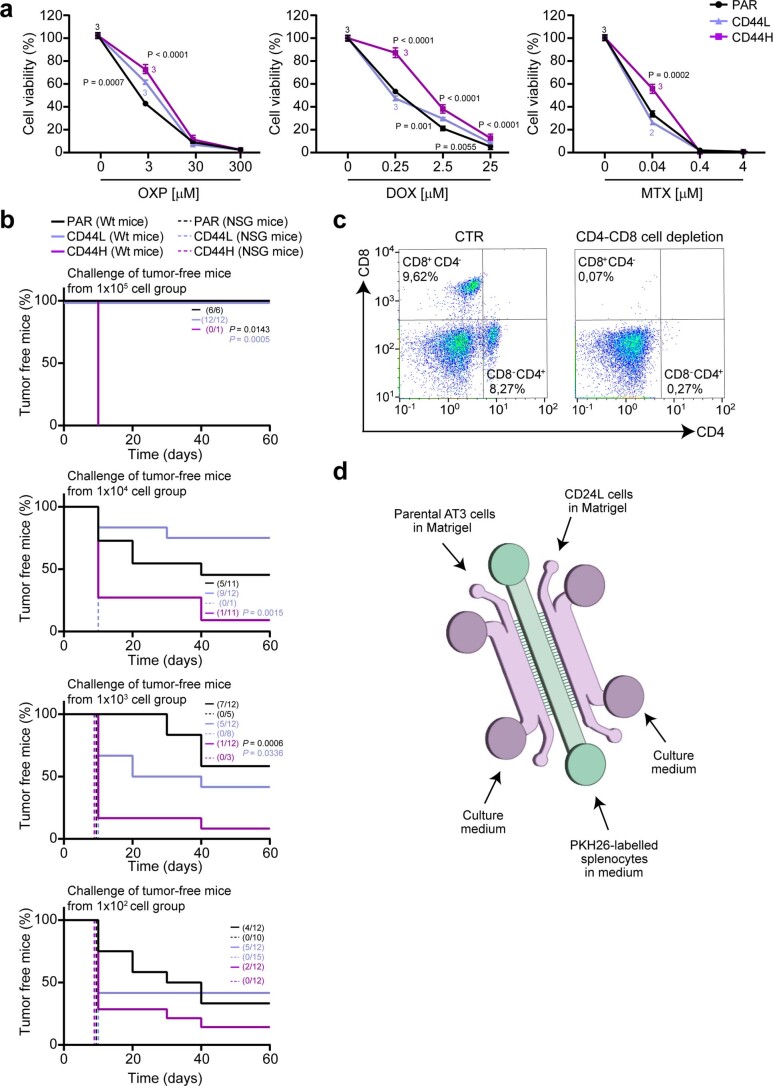
Extended Data Fig. 4. Characterization of cancer stem cells (CSCs) enriched by type I interferons (IFN-I).
(a) Evaluation of cell proliferation/viability by CellTiter-Glo® assay in parental (PAR) and FACS-isolated CD133+CD24+CD44+low (CD44L) and CD133+CD24+CD44+high (CD44H) MCA205 cells (upon enrichment via IFN-I administration) treated for 72 h with oxaliplatin (OXP), doxorubicin (DOX) and mitoxantrone (MTX) as indicated. Results are reported as mean ± s.e.m., n = 3 biologically independent experiments. (b) In vivo evaluation of the prophylactic potential of PAR MCA205 and immunogenic cell death (ICD)-induced CSCs by using immunocompetent C57Bl/6J (Wild-type/Wt) mice or immunodeficient NSG mice that rejected the injections with PAR, CD44H and CD44L cells at the indicated dose in the experiment reported in Fig. 4b and rechallenging the animals with 1 × 105 PAR MCA205 in the other flank. The percentage of tumor-free mice is shown. (c) Ex vivo flow cytometric analysis of CD4 and CD8 expression in splenocytes from C57Bl/6J mice treated intraperitoneally with vehicle (CTR) or 200 µg/mouse of anti-CD4 and anti-CD8 (200 µg/mouse at day -1 and then every 4 days for 2 weeks). One representative experiment out of two is shown. (d) Schematic representation of ‘competition’ microfluidic devices. CD24L, CD133+CD44+CD24+low. (a) Ordinary one-way ANOVA test followed by Bonferroni’s correction. (b) Log-rank (Mantel-Cox) test.