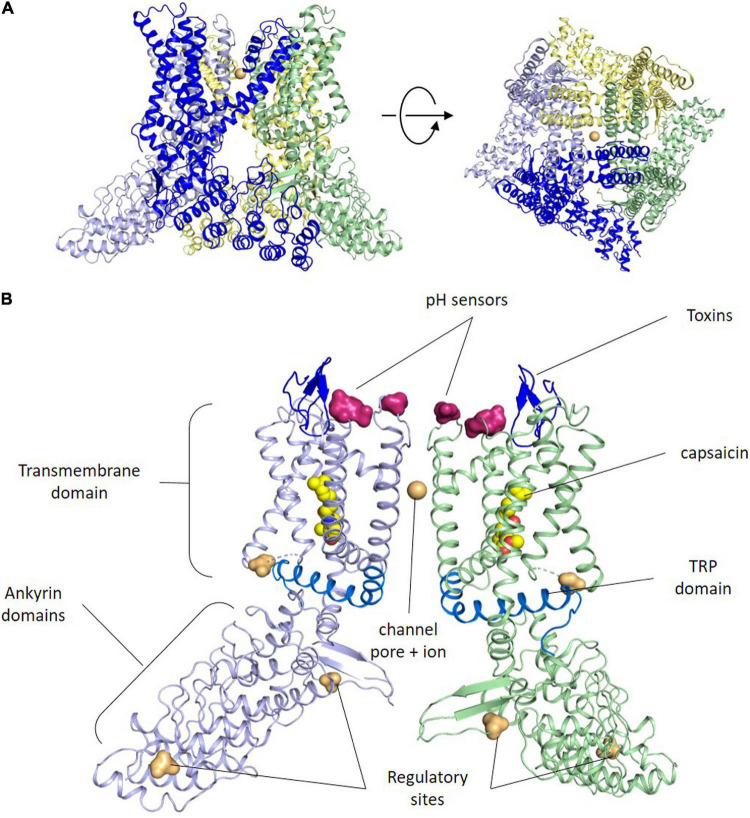
FIGURE 1.
TRPV1 structure. (A) Side and upper views of tetrameric arrangement of the full human TRPV1 in the closed state. Each subunit of the TRPV1 channel is made up of six transmembrane domains. The pore loop is located between the S5 and S6 segments. Both the N and C termini are located Intracellularly. Sodium ions are shown as orange spheres. Contacts between monomers occur mainly in the membrane domain and at the C-terminus coiled–coil domain that lies around the central axis and surrounded by the N terminus. (B) Side view of the ribbon structural model of two opposite monomers of TRPV1 channel. The other two monomers are not shown for clarity. Amino acid residues specific for capsaicin and proton activation, as well as residues important for toxins binding, phosphorylation by protein kinases A and C (PKA, PKC) and Ca2+-calmodulin-dependent kinase II (CaMKII) kinases are highlighted at both intra-cytoplasmic regions.