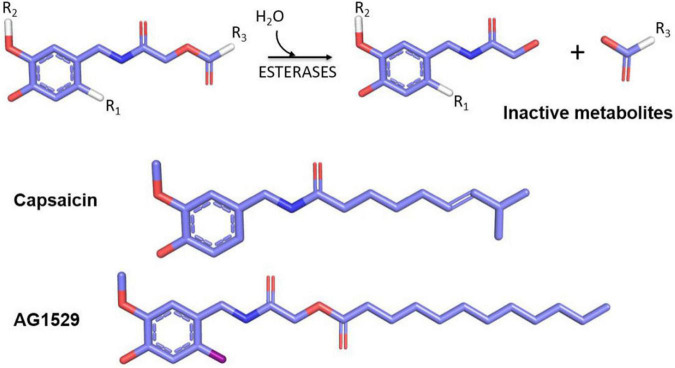
FIGURE 5.
Soft drugs derived from capsaicin (upper part) have an ester bond sensitive to hydrolysis by skin esterases that produce an inert and easily disposable metabolite (vanillylacetamide) and a lipid that can be assimilated by cells. The inclusion of an iodine atom at the R1 position generates TRPV1 antagonists, and positions R2 and R3 may be used to increase the library chemical diversity. In the lower part, the comparative chemical structures between capsaicin and the selected AG1529 for further clinical development are shown.