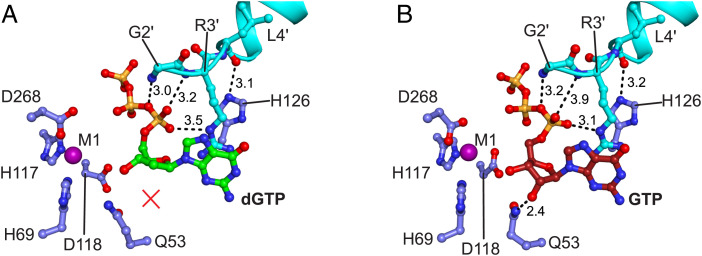
Fig. 5.
The Gp1.2 N terminus interacts with dGTP/GTP ligands and critical Dgt residues in the active site. Image generated in PyMOL. (A) Active site of the Dgt–Gp1.2–dGTP structure, with Dgt residues shown in blue, Gp1.2 in cyan, and dGTP in green and all colored by heteroatom. Dashed lines are the interactions with Gp1.2 that replace productive substrate interactions. dGTP lacks the interaction with Q53 (red X) in the ternary complex. (B) Active site of the Dgt–Gp1.2–GTP structure, with Dgt residues shown in blue, Gp1.2 in cyan, and GTP in red and all colored by heteroatom. Dashed lines are the interactions between Gp1.2 and GTP equivalent to the interactions in A, as well as the productive interaction between Q53 and the GTP 2′-OH.