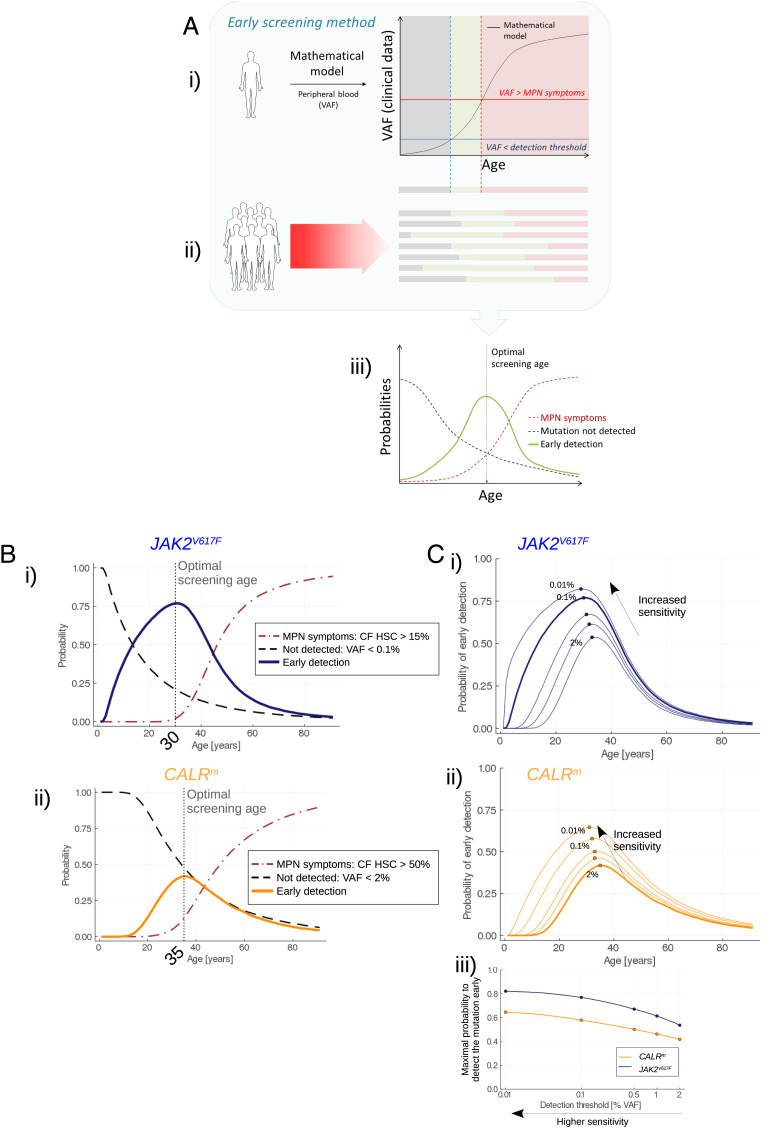
Fig. 3.
Early screening. (A) Overview of the method for determining the optimal screening age. i) One trajectory of the stochastic model (after its calibration from actual observations) corresponds to the disease expansion in an arbitrary (randomly sampled) patient. From the model, we obtain the progression of the VAF in mature cells over the years. Three periods in the patient’s life can be considered. First, the VAF is lower than a detection threshold (gray area); the malignant clone has already begun expanding but is still undetectable. Then (green area), the mutation becomes detectable still with a sufficiently low CF so that there is a low risk of MPN symptoms. Theoretically, it would be the appropriate period for the early screening of this particular patient. Eventually, the malignant clone continues to expand; the VAF exceeds a threshold above which there is a risk of MPN symptoms (red area): screening would be too late. ii) Because of the heterogeneity between patients, the three previously defined periods [mutation not detected (gray); early detection (green); and MPN symptoms (red)] are different between individuals. iii) Considering a high number of MPN patients, we obtain as functions of age the frequencies (or probabilities) of early screening (green line), to not detect the mutation (dashed gray line), or to have MPN symptoms (red dashed line). The probability of early screening reaches a maximum at the optimal screening age, that is, the age at which it would be optimal to test the population for the considered mutation. The value reached at the optimal screening age corresponds to the highest proportion of patients that would be detected early enough (according to our mathematical model and its calibration). The higher this value, the more efficient the screening. (B) Evolution of the probability of early detection (solid line, blue for JAK2V617F (i) and orange for CALRm (ii)), false negative rate (dashed line), and too late detection (dash-dotted line) when testing the population for the JAK2V617F (i) or CALRm (ii) mutation at different ages. Here, we consider that CALRm mutation is detected in mature cells for a VAF higher than 2% (sizing CALR) and that JAK2V617F is detected for a VAF higher than 0.1% (allele-specific PCR). (C) Impact of the sensitivity of the techniques for detecting JAK2V617F (i) or CALRm (ii) mutations. As expected, higher sensitivities increase the probability to detect the mutations, at earlier ages. In (iii), we compute the maximal probability to detect the mutation (at the corresponding optimal screening age) for different VAF thresholds ranging from 2 to 0.01% (x-axis in log scale).