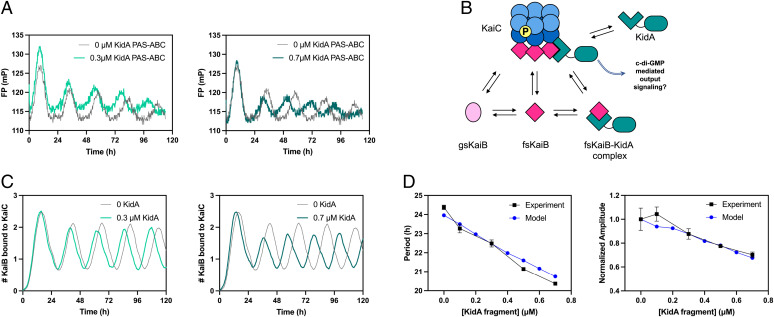
Fig. 4.
In vitro and in silico reconstitution of the KidA period-shortening effect. (A) In vitro reconstitution of the period-shortening effect of KidA. The KidA PAS-ABC fragment was added to a standard mixture of KaiA, KaiB, and KaiC. Oscillations were measured by fluorescence polarization of labeled KaiB (averaged traces shown from duplicate experiments). (B) Cartoon overview of a mathematical model based on the hypothesis that KidA stabilizes the fold-switched form of KaiB by direct binding. The Paijmans model was modified to explicitly describe the interconversion between the ground-state and fold-switched state of KaiB.The protein complexes and reaction arrows shown were added to allow KidA to bind fsKaiB (SI Appendix). (C) Time course of oscillations of KaiC-bound KaiB molecules per hexamer in the mathematical model. Model parameters were chosen to have period-dependence close to the experimental value. (D) Plots of the period and amplitude of the fluorescence polarization rhythm (as shown in Fig. 4A) as a function of [KidA] and the predictions from the model with the same parameter set (as shown in Fig. 4C). (Left) Period and (Right) amplitude normalized by dividing by the amplitude value at 0 µM KidA. Mean values are plotted, and the error bars show the SD (Experimental n = 2, Model n = 20).