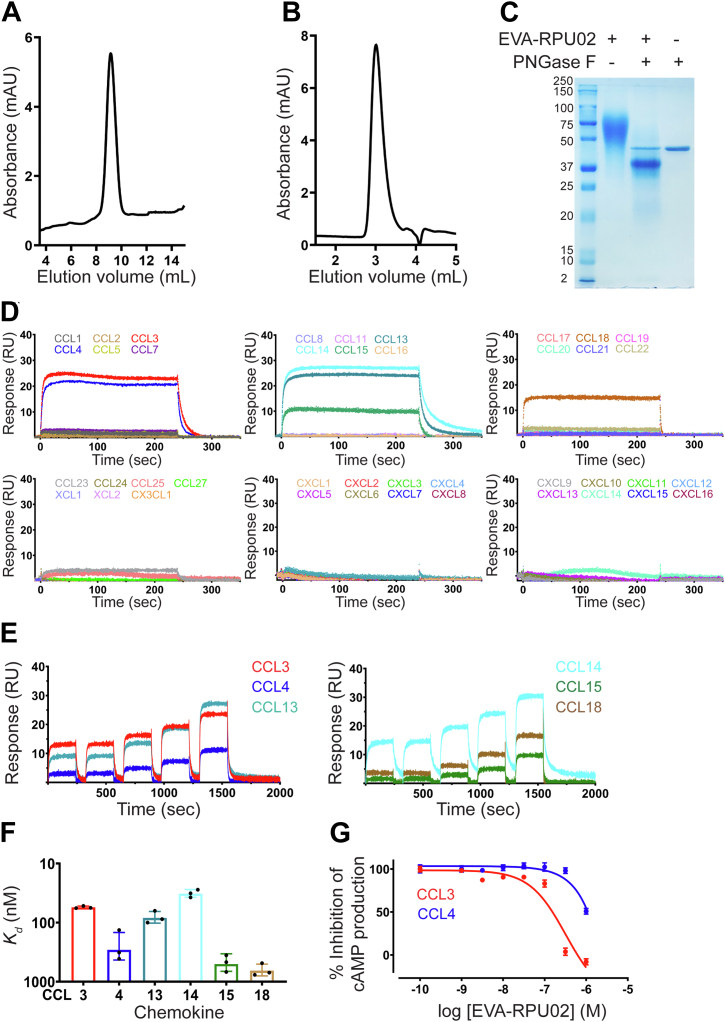
Figure 1.
EVA-R expressed in mammalian cells binds CC chemokines and inhibits their function.A and B, analytical reversed-phase high performance liquid chromatogram (A) and analytical size-exclusion chromatogram (B) of purified EVA-R. C, Coomassie blue–stained SDS-polyacrylamide gel showing purified EVA-R before and after PNGase F treatment: lane 1, protein molecular weight markers (labeled in kDa); lane 2, EVA-R after purification by size-exclusion chromatography; lane 3, purified EVA-R after treatment with PNGase F; lane 4, PNGase F alone. D, representative surface plasmon resonance sensorgrams showing binding of immobilized EVA-R to six CC chemokines (CCL3, CCL4, CCL13, CCL14, CCL15, and CCL18) but not to other CC or any CXC, CX3C, or XC chemokines, at 500 nM concentration. E, representative surface plasmon resonance sensorgrams showing binding of immobilized EVA-R to five different concentrations of the indicated chemokines (31.25, 63.5, 125, 250, and 500 nM). F, binding affinities of EVA-R for CC chemokines, measured by SPR (mean ± SD from three independent experiments). G, concentration response curves showing the inhibition of chemokines CCL3 and CCL4 by EVA-R. FlpInCHO cells stably expressing CCR5 and transfected with the cAMP biosensor CAMYEL, were treated with coelenterazine-H (5 μM, 10 min), followed by forskolin (10 μM, 10 min), followed by CCL3 (60 nM) or CCL4 (80 nM), either alone or pre-incubated with the indicated concentrations of EVA-R. Inhibition of forskolin-induced cAMP production was detected 10 min after chemokine addition. Data are presented as mean ± SEM from three independent experiments. cAMP, cyclic AMP; SPR, surface plasmon resonance.